Topical administration of somatostatin prevents retinal neurodegeneration in experimental diabetes
- PMID: 23474487
- PMCID: PMC3712066
- DOI: 10.2337/db12-0926
Topical administration of somatostatin prevents retinal neurodegeneration in experimental diabetes
Abstract
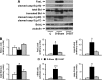
Retinal neurodegeneration is an early event in the pathogenesis of diabetic retinopathy (DR). Somatostatin (SST) is an endogenous neuroprotective peptide that is downregulated in the diabetic eye. The aim of the study was to test the usefulness of topical administration of SST in preventing retinal neurodegeneration. For this purpose, rats with streptozotocin-induced diabetes mellitus (STZ-DM) were treated with either SST eye drops or vehicle for 15 days. Nondiabetic rats treated with vehicle served as a control group. Functional abnormalities were assessed by electroretinography (ERG), and neurodegeneration was assessed by measuring glial activation and the apoptotic rate. In addition, proapoptotic (FasL, Bid, and activation of caspase-8 and caspase-3) and survival signaling pathways (BclxL) were examined. Intraretinal concentrations of glutamate and its main transporter glutamate/aspartate transporter (GLAST) were also determined. Treatment with SST eye drops prevented ERG abnormalities, glial activation, apoptosis, and the misbalance between proapoptotic and survival signaling detected in STZ-DM rats. In addition, SST eye drops inhibited glutamate accumulation in the retina and GLAST downregulation induced by diabetes mellitus. We conclude that topical administration of SST has a potent effect in preventing retinal neurodegeneration induced by diabetes mellitus. In addition, our findings open up a new preventive pharmacological strategy targeted to early stages of DR.
Figures






References
-
- Lorenzi M, Gerhardinger C. Early cellular and molecular changes induced by diabetes in the retina. Diabetologia 2001;44:791–804 - PubMed
-
- Barber AJ. A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry 2003;27:283–290 - PubMed
-
- Asnaghi V, Gerhardinger C, Hoehn T, Adeboje A, Lorenzi M. A role for the polyol pathway in the early neuroretinal apoptosis and glial changes induced by diabetes in the rat. Diabetes 2003;52:506–511 - PubMed
-
- Antonetti DA, Barber AJ, Bronson SK, et al. JDRF Diabetic Retinopathy Center Group Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes 2006;55:2401–2411 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

