Hyposensitivity to gamma-aminobutyric acid in the ventral tegmental area during alcohol withdrawal: reversal by histone deacetylase inhibitors
- PMID: 23474591
- PMCID: PMC3717553
- DOI: 10.1038/npp.2013.65
Hyposensitivity to gamma-aminobutyric acid in the ventral tegmental area during alcohol withdrawal: reversal by histone deacetylase inhibitors
Abstract
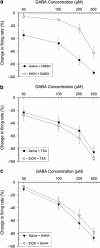
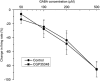
Putative dopaminergic (pDAergic) ventral tegmental area (VTA) neurons have an important role in alcohol addiction. Acute ethanol increases the activity of pDAergic neurons, and withdrawal from repeated ethanol administration produces a decreased sensitivity of pDAergic VTA neurons to GABA. Recent studies show that behavioral changes induced by chronic alcohol are reversed by inhibitors of histone deacetylases (HDACs). Whether HDAC-induced histone modifications regulate changes in GABA sensitivity of VTA pDAergic neurons during withdrawal is unknown. Here, we investigated modulation of withdrawal-induced changes in GABA sensitivity of pDAergic VTA neurons by HDAC inhibitors (HDACi), and also measured the levels of HDAC2, histone (H3-K9) acetylation, and GABA-Aα1 receptor (GABA (A-α1) R) subunit in VTA during ethanol withdrawal. Mice were injected intraperitoneally (ip) with either ethanol (3.5 g/kg) or saline twice daily for 3 weeks. In recordings from pDAergic VTA neurons in brain slices from ethanol-withdrawn mice, sensitivity to GABA (50-500 μM) was reduced. In brain slices from ethanol-withdrawn mice incubated with the HDACi SAHA (vorinostat) or trichostatin A (TSA) for 2 h, the hyposensitivity of pDAergic VTA neurons to GABA was significantly attenuated. There was no effect of TSA or SAHA on GABA sensitivity of pDAergic VTA neurons from saline-treated mice. In addition, ethanol withdrawal was associated with an increase in levels of HDAC2 and a decrease in histone (H3-K9) acetylation and levels of GABA (A-α1) R subunits in the VTA. Therefore, blockade of upregulation of HDAC2 by HDACi normalizes GABA hyposensitivity of pDAergic neurons developed during withdrawal after chronic ethanol treatment, which suggests the possibility that inhibition of HDACs can reverse ethanol-induced neuroadaptational changes in reward circuitry.
Figures



References
-
- Albanese A, Minciacchi D. Organization of the ascending projections from the ventral tegmental area: a multiple fluorescent retrograde tracer study in the rat. J Comp Neurol. 1983;216:406–420. - PubMed
-
- Bailey CP, Manley SJ, Watson WP, Wonnacott S, Molleman A, Little HJ. Chronic ethanol administration alters activity in ventral tegmental area neurons after cessation of withdrawal hyperexcitability. Brain Res. 1998;803:144–152. - PubMed
-
- Brodie MS. Increased ethanol excitation of dopaminergic neurons of the ventral tegmental area after chronic ethanol treatment. Alcohol Clin Exp Res. 2002;26:1024–1030. - PubMed
-
- Brodie MS, McElvain MA, Bunney EB, Appel SB. Pharmacological reduction of small conductance calcium-activated potassium current (SK) potentiates the excitatory effect of ethanol on ventral tegmental area dopamine neurons. J Pharmacol Exp Ther. 1999a;290:325–333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

