Canonical and non-canonical NF-κB signaling promotes breast cancer tumor-initiating cells
- PMID: 23474754
- PMCID: PMC4425414
- DOI: 10.1038/onc.2013.64
Canonical and non-canonical NF-κB signaling promotes breast cancer tumor-initiating cells
Abstract
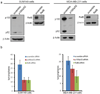
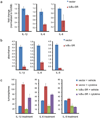
Tumor-initiating cells (TICs) are a sub-population of cells that exhibit a robust ability to self-renew and contribute to the formation of primary tumors, the relapse of previously treated tumors and the development of metastases. TICs have been identified in various tumors including those of the breast, and are particularly enriched in the basal-like and claudin-low subtypes of breast cancer. The signaling pathways that contribute to the function and maintenance of TICs are under intense study. We explored the potential involvement of the nuclear factor-κB (NF-κB) family of transcription factors in TICs in cell lines that are representative of basal-like and claudin-low breast cancer. NF-κB was found to be activated in breast cancer cells that form tumorspheres efficiently. Moreover, both canonical and non-canonical NF-κB signaling is required for these cells to self-renew in vitro and to form xenograft tumors efficiently in vivo using limiting dilutions of cells. Consistent with this fact, canonical and non-canonical NF-κB signaling is activated in TICs isolated from breast cancer cell lines. Experimental results indicate that NF-κB promotes the function of TICs by stimulating epithelial-to-mesenchymal transition and by upregulating the expression of the inflammatory cytokines interleukin-1β and interleukin-6. The results suggest the use of NF-κB inhibitors for clinical therapy of certain breast cancers.
Conflict of interest statement
Figures



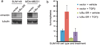
References
-
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. - PubMed
-
- Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. - PubMed
-
- Lawson JC, Blatch GL, Edkins AL. Cancer stem cells in breast cancer and metastasis. Breast Cancer Res Treat. 2009;2:241–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

