Protein kinase Cδ is required for ErbB2-driven mammary gland tumorigenesis and negatively correlates with prognosis in human breast cancer
- PMID: 23474764
- PMCID: PMC4292929
- DOI: 10.1038/onc.2013.59
Protein kinase Cδ is required for ErbB2-driven mammary gland tumorigenesis and negatively correlates with prognosis in human breast cancer
Abstract
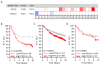
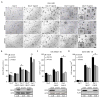
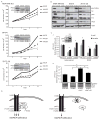
Protein kinase C δ (PKCδ) regulates apoptosis in the mammary gland, however, the functional contribution of PKCδ to the development or progression of breast cancer has yet to be determined. Meta-analysis of ErbB2-positive breast cancers shows increased PKCδ expression, and a negative correlation between PKCδ expression and prognosis. Here, we present in-vivo evidence that PKCδ is essential for the development of mammary gland tumors in a ErbB2-overexpressing transgenic mouse model, and in-vitro evidence that PKCδ is required for proliferative signaling downstream of the ErbB2 receptor. Mouse mammary tumor virus (MMTV)-ErbB2 mice lacking PKCδ (δKO) have increased tumor latency compared with MMTV-ErbB2 wild-type (δWT) mice, and the tumors show a dramatic decrease in Ki-67 staining. To explore the relationship between PKCδ and ErbB2-driven proliferation more directly, we used MCF-10A cells engineered to express a synthetic ligand-inducible form of the ErbB2 receptor. Depletion of PKCδ with short hairpin RNA inhibited ligand-induced growth in both two-dimensional (2D) (plastic) and three-dimensional (3D) (Matrigel) culture, and correlated with decreased phosphorylation of the ErbB2 receptor and reduced activation of Src and MAPK/ERK pathways. Similarly, in human breast cancer cell lines in which ErbB2 is overexpressed, depletion of PKCδ suppresses proliferation, Src and ERK activation. PKCδ appears to drive proliferation through the formation of an active ErbB2/PKCδ/Src signaling complex, as depletion of PKCδ disrupts association of Src with the ErbB2 receptor. Taken together, our studies present the first evidence that PKCδ is a critical regulator of ErbB2-mediated tumorigenesis, and suggest further investigation of PKCδ as a target in ErbB2-positive breast cancer.
Conflict of interest statement
Figures


References
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. - PubMed
-
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–12. - PubMed
-
- Jones JT, Akita RW, Sliwkowski MX. Binding specificities and affinities of egf domains for ErbB receptors. FEBS Lett. 1999;447:227–31. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

