Long-term follow up of patients proceeding to transplant using plerixafor mobilized stem cells and incidence of secondary myelodysplastic syndrome/AML
- PMID: 23474805
- PMCID: PMC3773813
- DOI: 10.1038/bmt.2013.10
Long-term follow up of patients proceeding to transplant using plerixafor mobilized stem cells and incidence of secondary myelodysplastic syndrome/AML
Abstract
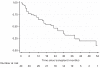
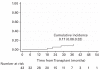
We report the long-term follow up of 49 patients (pts) enrolled on plerixafor compassionate use protocol. Thirty-seven pts (76%) had failed one previous mobilization attempt, while 12 (24%) had failed two or more previous attempts. Using the combination of plerixafor and granulocyte colony-stimulating factor, we collected2.5 × 10(6) CD34+cells/kg in 33 pts (67%). Forty-three of the 49 pts proceeded to an auto-SCT (ASCT). The median days to WBC and platelet engraftment were 11 (range, 9-13 days) and 16 (range, 11-77 days) days post ASCT, respectively. The median WBC count, Hb and platelet counts 1 year after ASCT were 4.7 × 10(9)/L, 12.2 g/dL and 109 × 10(9)/L, respectively. With median follow up of 42 months (range <1-54 months), 21 pts had evidence of disease progression. Five pts developed myelodysplastic syndrome (MDS)/AML at median of 29 months post ASCT. The cumulative incidence of MDS/AML at 42 months was 17% (95% confidence interval, 6 to 32%). Development of secondary MDS/AML in pts proceeding to ASCT after plerixafor mobilization needs to be studied further in a larger cohort.
Trial registration: ClinicalTrials.gov NCT00291811.
Figures
Similar articles
-
Plerixafor and G-CSF for PBSC mobilization in patients with lymphoma who failed previous attempts with G-CSF and chemotherapy: a REL (Rete Ematologica Lombarda) experience.Leuk Res. 2011 Jun;35(6):712-4. doi: 10.1016/j.leukres.2010.12.036. Epub 2011 Jan 26. Leuk Res. 2011. PMID: 21276613 Clinical Trial.
-
Risk factors before autologous stem-cell transplantation for lymphoma predict for secondary myelodysplasia and acute myelogenous leukemia.J Clin Oncol. 2006 Aug 1;24(22):3604-10. doi: 10.1200/JCO.2006.06.0673. J Clin Oncol. 2006. PMID: 16877727
-
Biosimilar Filgrastim (Tevagrastim, XMO2) for Allogeneic Hematopoietic Stem Cell Mobilization and Transplantation in Patients with Acute Myelogenous Leukemia/Myelodysplastic Syndromes.Biol Blood Marrow Transplant. 2016 Feb;22(2):277-283. doi: 10.1016/j.bbmt.2015.08.033. Epub 2015 Sep 4. Biol Blood Marrow Transplant. 2016. PMID: 26343949
-
Plerixafor injection: a hematopoietic stem cell mobilizer in non-Hodgkin lymphoma and multiple myeloma.Expert Rev Hematol. 2016 Aug;9(8):723-32. doi: 10.1080/17474086.2016.1208082. Epub 2016 Jul 15. Expert Rev Hematol. 2016. PMID: 27355397 Review.
-
Plerixafor: A chemokine receptor-4 antagonist for mobilization of hematopoietic stem cells for transplantation after high-dose chemotherapy for non-Hodgkin's lymphoma or multiple myeloma.Clin Ther. 2010 May;32(5):821-43. doi: 10.1016/j.clinthera.2010.05.007. Clin Ther. 2010. PMID: 20685493 Review.
Cited by
-
Hematopoietic progenitor cell collection after autologous transplant for multiple myeloma: low platelet count predicts for poor collection and sole use of resulting graft enhances risk of myelodysplasia.Leukemia. 2014 Apr;28(4):888-93. doi: 10.1038/leu.2013.214. Epub 2013 Jul 15. Leukemia. 2014. PMID: 23852547 Free PMC article.
-
Use of Plerixafor for Stem Cell Mobilization in the Setting of Autologous and Allogeneic Stem Cell Transplantations: An Update.J Blood Med. 2021 Jun 2;12:403-412. doi: 10.2147/JBM.S307520. eCollection 2021. J Blood Med. 2021. PMID: 34104027 Free PMC article. Review.
-
Hepatocellular Carcinoma: Old and Emerging Therapeutic Targets.Cancers (Basel). 2024 Feb 23;16(5):901. doi: 10.3390/cancers16050901. Cancers (Basel). 2024. PMID: 38473265 Free PMC article. Review.
-
Chemokine receptor 4 targeted protein MRI contrast agent for early detection of liver metastases.Sci Adv. 2020 Feb 7;6(6):eaav7504. doi: 10.1126/sciadv.aav7504. eCollection 2020 Feb. Sci Adv. 2020. PMID: 32083172 Free PMC article.
-
Recent Advances in CXCL12/CXCR4 Antagonists and Nano-Based Drug Delivery Systems for Cancer Therapy.Pharmaceutics. 2022 Jul 25;14(8):1541. doi: 10.3390/pharmaceutics14081541. Pharmaceutics. 2022. PMID: 35893797 Free PMC article. Review.
References
-
- Attal M, Harousseau JL, Facon T, Guilhot F, Doyen C, Fuzibet JG, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–2502. - PubMed
-
- Philip T, Chauvin F, Armitage J, Bron D, Hagenbeek A, Biron P, et al. Parma international protocol: pilot study of DHAP followed by involved-field radio-therapy and BEAC with autologous bone marrow transplantation. Blood. 1991;77:1587–1592. - PubMed
-
- Allan DS, Keeney M, Howson-Jan K, Popma J, Weir K, Bhatia M, et al. Number of viable CD34(+) cells reinfused predicts engraftment in autologous hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;29:967–972. - PubMed
-
- Bensinger W, DiPersio JF, McCarty JM. Improving stem cell mobilization strategies: future directions. Bone Marrow Transplant. 2009;43:181–195. - PubMed
-
- Villalon L, Odriozola J, Larana JG, Zamora C, Perez de Oteyza J, Jodra MH, et al. Autologous peripheral blood progenitor cell transplantation with <2 × 10(6) CD34(+)/kg: an analysis of variables concerning mobilisation and engraftment. Hematol J. 2000;1:374–381. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous