Comparison of sterol import under aerobic and anaerobic conditions in three fungal species, Candida albicans, Candida glabrata, and Saccharomyces cerevisiae
- PMID: 23475705
- PMCID: PMC3647772
- DOI: 10.1128/EC.00345-12
Comparison of sterol import under aerobic and anaerobic conditions in three fungal species, Candida albicans, Candida glabrata, and Saccharomyces cerevisiae
Abstract
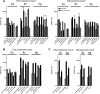
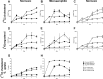
Sterol import has been characterized under various conditions in three distinct fungal species, the model organism Saccharomyces cerevisiae and two human fungal pathogens Candida glabrata and Candida albicans, employing cholesterol, the sterol of higher eukaryotes, as well as its fungal equivalent, ergosterol. Import was confirmed by the detection of esterified cholesterol within the cells. Comparing the three fungal species, we observe sterol import under three different conditions. First, as previously well characterized, we observe sterol import under low oxygen levels in S. cerevisiae and C. glabrata, which is dependent on the transcription factor Upc2 and/or its orthologs or paralogs. Second, we observe sterol import under aerobic conditions exclusively in the two pathogenic fungi C. glabrata and C. albicans. Uptake emerges during post-exponential-growth phases, is independent of the characterized Upc2-pathway and is slower compared to the anaerobic uptake in S. cerevisiae and C. glabrata. Third, we observe under normoxic conditions in C. glabrata that Upc2-dependent sterol import can be induced in the presence of fetal bovine serum together with fluconazole. In summary, C. glabrata imports sterols both in aerobic and anaerobic conditions, and the limited aerobic uptake can be further stimulated by the presence of serum together with fluconazole. S. cerevisiae imports sterols only in anaerobic conditions, demonstrating aerobic sterol exclusion. Finally, C. albicans imports sterols exclusively aerobically in post-exponential-growth phases, independent of Upc2. For the first time, we provide direct evidence of sterol import into the human fungal pathogen C. albicans, which until now was believed to be incapable of active sterol import.
Figures




References
-
- Jacquier N, Schneiter R. 2012. Mechanisms of sterol uptake and transport in yeast. J. Steroid Biochem. Mol. Biol. 129:70–78 - PubMed
-
- Raychaudhuri S, Prinz WA. 2006. Uptake and trafficking of exogenous sterols in Saccharomyces cerevisiae. Biochem. Soc. Trans. 34:359–362 - PubMed
-
- Schneiter R. 2007. Intracellular sterol transport in eukaryotes, a connection to mitochondrial function? Biochimie 89:255–259 - PubMed
-
- Schneiter R, Brugger B, Sandhoff R, Zellnig G, Leber A, Lampl M, Athenstaedt K, Hrastnik C, Eder S, Daum G, Paltauf F, Wieland FT, Kohlwein SD. 1999. Electrospray ionization tandem mass spectrometry (ESI-MS/MS) analysis of the lipid molecular species composition of yeast subcellular membranes reveals acyl chain-based sorting/remodeling of distinct molecular species en route to the plasma membrane. J. Cell Biol. 146:741–754 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

