Hydrogen gas reduces hyperoxic lung injury via the Nrf2 pathway in vivo
- PMID: 23475767
- PMCID: PMC3652058
- DOI: 10.1152/ajplung.00164.2012
Hydrogen gas reduces hyperoxic lung injury via the Nrf2 pathway in vivo
Abstract
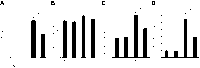
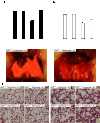
Hyperoxic lung injury is a major concern in critically ill patients who receive high concentrations of oxygen to treat lung diseases. Successful abrogation of hyperoxic lung injury would have a huge impact on respiratory and critical care medicine. Hydrogen can be administered as a therapeutic medical gas. We recently demonstrated that inhaled hydrogen reduced transplant-induced lung injury and induced heme oxygenase (HO)-1. To determine whether hydrogen could reduce hyperoxic lung injury and investigate the underlying mechanisms, we randomly assigned rats to four experimental groups and administered the following gas mixtures for 60 h: 98% oxygen (hyperoxia), 2% nitrogen; 98% oxygen (hyperoxia), 2% hydrogen; 98% balanced air (normoxia), 2% nitrogen; and 98% balanced air (normoxia), 2% hydrogen. We examined lung function by blood gas analysis, extent of lung injury, and expression of HO-1. We also investigated the role of NF-E2-related factor (Nrf) 2, which regulates HO-1 expression, by examining the expression of Nrf2-dependent genes and the ability of hydrogen to reduce hyperoxic lung injury in Nrf2-deficient mice. Hydrogen treatment during exposure to hyperoxia significantly improved blood oxygenation, reduced inflammatory events, and induced HO-1 expression. Hydrogen did not mitigate hyperoxic lung injury or induce HO-1 in Nrf2-deficient mice. These findings indicate that hydrogen gas can ameliorate hyperoxic lung injury through induction of Nrf2-dependent genes, such as HO-1. The findings suggest a potentially novel and applicable solution to hyperoxic lung injury and provide new insight into the molecular mechanisms and actions of hydrogen.
Keywords: NF-E2-related factor 2; heme oxygenase; hydrogen; inflammation.
Figures
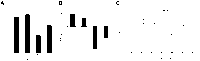





References
-
- Alam J, Cook JL. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am J Respir Cell Mol Biol 36: 166–174, 2007 - PubMed
-
- Alam J, Cook JL. Transcriptional regulation of the heme oxygenase-1 gene via the stress response element pathway. Curr Pharm Des 9: 2499–2511, 2003 - PubMed
-
- Altemeier WA, Sinclair SE. Hyperoxia in the intensive care unit: why more is not always better. Curr Opin Crit Care 13: 73–78, 2007 - PubMed
-
- Awasthi S, Gyurasics A, Knight SA, Welty SE, Smith CV. Protein oxidation biomarkers in hyperoxic lung injury in rats: effects of U-74389. Toxicol Lett 95: 47–61, 1998 - PubMed
-
- Baird L, Dinkova-Kostova AT. The cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol 85: 241–272, 2011 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

