A mutant with aberrant extracellular LcrV-YscF interactions fails to form pores and translocate Yop effector proteins but retains the ability to trigger Yop secretion in response to host cell contact
- PMID: 23475976
- PMCID: PMC3650545
- DOI: 10.1128/JB.02011-12
A mutant with aberrant extracellular LcrV-YscF interactions fails to form pores and translocate Yop effector proteins but retains the ability to trigger Yop secretion in response to host cell contact
Abstract
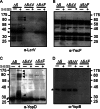
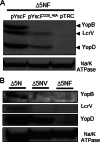
The plasmid-encoded type three secretion system (TTSS) of Yersinia spp. is responsible for the delivery of effector proteins into cells of the innate immune system, where these effectors disrupt the target cells' activity. Successful translocation of effectors into mammalian cells requires Yersinia to both insert a translocon into the host cell membrane and sense contact with host cells. To probe the events necessary for translocation, we investigated protein-protein interactions among TTSS components of the needle-translocon complex using a chemical cross-linking-based approach. We detected extracellular protein complexes containing YscF, LcrV, and YopD that were dependent upon needle formation. The formation of these complexes was evaluated in a secretion-competent but translocation-defective mutant, the YscFD28AD46A strain (expressing YscF with the mutations D28A and D46A). We found that one of the YscF and most of the LcrV and YopD cross-linked complexes were nearly absent in this mutant. Furthermore, the YscFD28AD46A strain did not support YopB insertion into mammalian membranes, supporting the idea that the LcrV tip complex is required for YopB insertion and translocon formation. However, the YscFD28AD46A strain did secrete Yops in the presence of host cells, indicating that a translocation-competent tip complex is not required to sense contact with host cells to trigger Yop secretion. In conclusion, in the absence of cross-linkable LcrV-YscF interactions, translocon insertion is abolished, but Yersinia still retains the ability to sense cell contact.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

