Idiosyncratic adverse drug reactions: current concepts
- PMID: 23476052
- PMCID: PMC3639727
- DOI: 10.1124/pr.113.007450
Idiosyncratic adverse drug reactions: current concepts
Abstract
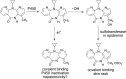
Idiosyncratic drug reactions are a significant cause of morbidity and mortality for patients; they also markedly increase the uncertainty of drug development. The major targets are skin, liver, and bone marrow. Clinical characteristics suggest that IDRs are immune mediated, and there is substantive evidence that most, but not all, IDRs are caused by chemically reactive species. However, rigorous mechanistic studies are very difficult to perform, especially in the absence of valid animal models. Models to explain how drugs or reactive metabolites interact with the MHC/T-cell receptor complex include the hapten and P-I models, and most recently it was found that abacavir can interact reversibly with MHC to alter the endogenous peptides that are presented to T cells. The discovery of HLA molecules as important risk factors for some IDRs has also significantly contributed to our understanding of these adverse reactions, but it is not yet clear what fraction of IDRs have a strong HLA dependence. In addition, with the exception of abacavir, most patients who have the HLA that confers a higher IDR risk with a specific drug will not have an IDR when treated with that drug. Interindividual differences in T-cell receptors and other factors also presumably play a role in determining which patients will have an IDR. The immune response represents a delicate balance, and immune tolerance may be the dominant response to a drug that can cause IDRs.
Figures


References
-
- Adam J, Eriksson KK, Schnyder B, Fontana S, Pichler WJ, Yerly D. (2012) Avidity determines T-cell reactivity in abacavir hypersensitivity. Eur J Immunol 42:1706–1716 - PubMed
-
- Aeby P, Sieber T, Beck H, Gerberick GF, Goebel C. (2009) Skin sensitization to p-phenylenediamine: the diverging roles of oxidation and N-acetylation for dendritic cell activation and the immune response. J Invest Dermatol 129:99–109 - PubMed
-
- Aithal GP, Ramsay L, Daly AK, Sonchit N, Leathart JB, Alexander G, Kenna JG, Caldwell J, Day CP. (2004) Hepatic adducts, circulating antibodies, and cytokine polymorphisms in patients with diclofenac hepatotoxicity. Hepatology 39:1430–1440 - PubMed
-
- Akdis CA, Akdis M. (2009) Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J Allergy Clin Immunol 123:735–748 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

