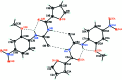
1-(2-Furo-yl)-3-(2-meth-oxy-4-nitro-phen-yl)thio-urea
- PMID: 23476526
- PMCID: PMC3588461
- DOI: 10.1107/S1600536813002894
1-(2-Furo-yl)-3-(2-meth-oxy-4-nitro-phen-yl)thio-urea
Abstract
The asymmetric unit of the title compound, C13H11N3O5S, contains two independent mol-ecules, which are linked by a pair of inter-molecular N-H⋯S hydrogen bonds, forming an R2(2)(8) ring motif. The central thio-urea core forms dihedral angles of 3.02 (12) and 14.00 (10)° with the essentially planar furoyl groups [maximum deviations = 0.030 (2) and 0.057 (2) Å] in the two mol-ecules and dihedral angles of 2.43 (13) and 8.03 (12)° with the benzene rings. The dihedral angles between the furoyl and benzene rings in the two mol-ecules are 3.97 (10) and 5.98 (9)°. The trans-cis geometry of the thio-urea group is stabilized by three intra-molecular N-H⋯O hydrogen bonds involving carbonyl and meth-oxy O atoms with the H atom of the cis-thio-amide group and between furan O atom and the other thio-amide H atom. There is also a weak intra-molecular C-H⋯S inter-action in each mol-ecule.
Figures
References
-
- Agilent (2012). CrysAlis PRO. Agilent Technologies, Yarnton, England.
-
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
-
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl. 34, 1555–1573.
-
- Clark, R. C. & Reid, J. S. (1995). Acta Cryst. A51, 887–897.
-
- Dai, M., Liang, B., Wang, C., Chen, J. & Yang, Z. (2004). Org. Lett. 6, 221–224. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources