{2,7-Dimeth-oxy-8-[4-(propan-2-yl-oxy)benzo-yl]naphthalen-1-yl}[4-(propan-2-yl-oxy)phen-yl]methanone
- PMID: 23476613
- PMCID: PMC3588464
- DOI: 10.1107/S1600536813004959
{2,7-Dimeth-oxy-8-[4-(propan-2-yl-oxy)benzo-yl]naphthalen-1-yl}[4-(propan-2-yl-oxy)phen-yl]methanone
Abstract
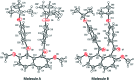
The title compound, C32H32O6, crystallized with two independent molecules in the asymmetric unit. Each molecule has essentially the same feature of non-coplanar aromatic rings whereby the two 4-isopropoxybenzoyl groups are twisted in a perpendicular manner to the naphthalene ring and oriented in the same direction (syn-orientation). The benzene rings of the aroyl groups make dihedral angles of 16.13 (7) and 25.31 (7)° in the two molecules. These benzene rings make dihedral angles of 88.38 (8) and 75.32 (7)° with the naphthalene ring system in one molecule, and 89.71 (7) and 82.11 (7)° in the other. In the crystal, mol-ecules are linked via C-H⋯O hydrogen bonds, forming a three-dimensional network. In one independent molecule, the 2-propyl groups of both isoprop-oxy groups are disordered over two positions with site occupancies of 0.512 (3) and 0.488 (3).
Figures

References
-
- Burnett, M. N. & Johnson, C. K. (1996). ORTEPIII Report ORNL-6895. Oak Ridgeational Laboratory, Tennessee, USA.
-
- Higashi, T. (1999). NUMABS Rigaku Corporation, Tokyo, Japan.
-
- Okamoto, A., Mitsui, R., Oike, H. & Yonezawa, N. (2011). Chem Lett 40, 1283-1284.
-
- Okamoto, A. & Yonezawa, N. (2009). Chem. Lett. 38, 914–915.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
