Phosphorylation and ionic strength alter the LRAP-HAP interface in the N-terminus
- PMID: 23477367
- PMCID: PMC3626292
- DOI: 10.1021/bi400071a
Phosphorylation and ionic strength alter the LRAP-HAP interface in the N-terminus
Abstract
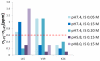
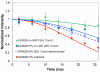
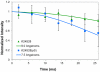
The conditions present during enamel crystallite development change dramatically as a function of time, including the pH, protein concentration, surface type, and ionic strength. In this work, we investigate the role that two of these changing conditions, pH and ionic strength, have in modulating the interaction of the amelogenin, LRAP, with hydroxyapatite (HAP). Using solid-state NMR dipolar recoupling and chemical shift data, we investigate the structure, orientation, and dynamics of three regions in the N-terminus of the protein: L(15) to V(19), V(19) to L(23), and K(24) to S(28). These regions are also near the only phosphorylated residue in the protein pS(16); therefore, changes in the LRAP-HAP interaction as a function of phosphorylation (LRAP(-P) vs LRAP(+P)) were also investigated. All of the regions and conditions studied for the surface immobilized proteins showed restricted motion, with indications of slightly more mobility under all conditions for L(15)(+P) and K(24)(-P). The structure and orientation of the LRAP-HAP interaction in the N-terminus of the phosphorylated protein is very stable to changing solution conditions. From REDOR dipolar recoupling data, the structure and orientation in the region L(15)V(19)(+P) did not change significantly as a function of pH or ionic strength. The structure and orientation of the region V(19)L(23)(+P) were also stable to changes in pH, with the only significant change observed at high ionic strength, where the region becomes extended, suggesting this may be an important region in regulating mineral development. Chemical shift studies also suggest minimal changes in all three regions studied for both LRAP(-P) and LRAP(+P) as a function of pH or ionic strength, and also reveal that K(24) has multiple resolvable resonances, suggestive of two coexisting structures. Phosphorylation also alters the LRAP-HAP interface. All of the three residues investigated (L(15), V(19), and K(24)) are closer to the surface in LRAP(+P), but only K(24)S(28) changes structure as a result of phosphorylation, from a random coil to a largely helical structure, and V(19)L(23) becomes more extended at high ionic strength when phosphorylated. These observations suggest that ionic strength and dephosphorylation may provide switching mechanisms to trigger a change in the function of the N-terminus during enamel development.
Figures


References
-
- Simmer JP, Fincham AG. Molecular mechanisms of dental enamel formation. Crit Rev Oral Biol Med. 1995;6:84–108. - PubMed
-
- Fincham AG, Moradian-Oldak J, Simmer JP. The Structural Biology of the Developing Dental Enamel Matrix. J. Struct. Biol. 1999;136:270–299. - PubMed
-
- Moradian-Oldak J, Tan J, Fincham AG. Interaction of amelogenin with hydroxyapatite crystals: an adherence effect through amelogenin molecular self-association. Biopolymers. 1998;46:225–238. - PubMed
-
- Shaw WJ, Campbell AA, Paine ML, Snead ML. The COOH terminus of the amelogenin, LRAP, is oriented next to the hydroxyapatite surface. J. Biol. Chem. 2004;279:40263–40266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

