Physicochemical characterization of airborne particulate matter at a mainline underground railway station
- PMID: 23477491
- PMCID: PMC3687366
- DOI: 10.1021/es304481m
Physicochemical characterization of airborne particulate matter at a mainline underground railway station
Abstract
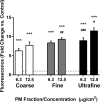
Underground railway stations are known to have elevated particulate matter (PM) loads compared to ambient air. As these particles are derived from metal-rich sources and transition metals may pose a risk to health by virtue of their ability to catalyze generation of reactive oxygen species (ROS), their potential enrichment in underground environments is a source of concern. Compared to coarse (PM10) and fine (PM2.5) particulate fractions of underground railway airborne PM, little is known about the chemistry of the ultrafine (PM0.1) fraction that may contribute significantly to particulate number and surface area concentrations. This study uses inductively coupled plasma mass spectrometry and ion chromatography to compare the elemental composition of size-fractionated underground PM with woodstove, roadwear generator, and road tunnel PM. Underground PM is notably rich in Fe, accounting for greater than 40% by mass of each fraction, and several other transition metals (Cu, Cr, Mn, and Zn) compared to PM from other sources. Importantly, ultrafine underground PM shows similar metal-rich concentrations as the coarse and fine fractions. Scanning electron microscopy revealed that a component of the coarse fraction of underground PM has a morphology indicative of generation by abrasion, absent for fine and ultrafine particulates, which may be derived from high-temperature processes. Furthermore, underground PM generated ROS in a concentration- and size-dependent manner. This study suggests that the potential health effects of exposure to the ultrafine fraction of underground PM warrant further investigation as a consequence of its greater surface area/volume ratio and high metal content.
Figures

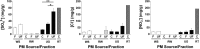

References
-
- Nieuwenhuijsen M. J.; Gomez-Perales J. E.; Colvile R. N. Levels of particulate air pollution, its elemental composition, determinants and health effects in metro systems. Atmos. Environ. 2007, 41377995–8006.
-
- Sitzmann B.; Kendall M.; Watt J.; Williams I. Characterisation of airborne particles in London by computer-controlled scanning electron microscopy. Sci. Total Environ. 1999, 2411–363–73.
-
- Bachoual R.; Boczkowski J.; Goven D.; Amara N.; Tabet L.; On D.; Lecon-Malas V.; Aubier M.; Lanone S. Biological effects of particles from the Paris subway system. Chem. Res. Toxicol. 2007, 20101426–1433. - PubMed
-
- Ripanucci G.; Grana M.; Vicentini L.; Magrini A.; Bergamaschi A. Dust in the underground railway tunnels of an Italian town. J. Occup. Environ. Hyg. 2006, 3116–25. - PubMed
-
- World Health Organization. Air Quality Guidelines: Global Update 2005: Particulate Matter, Ozone, Nitrogen Dioxide, and Sulfur Dioxide; World Health Organization: Copenhagen, Denmark, 2006; p 9.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

