The addition of amifostine to carboplatin and paclitaxel based chemoradiation in locally advanced non-small cell lung cancer: long-term follow-up of Radiation Therapy Oncology Group (RTOG) randomized trial 9801
- PMID: 23477890
- PMCID: PMC3646966
- DOI: 10.1016/j.lungcan.2013.02.008
The addition of amifostine to carboplatin and paclitaxel based chemoradiation in locally advanced non-small cell lung cancer: long-term follow-up of Radiation Therapy Oncology Group (RTOG) randomized trial 9801
Abstract
Introduction: We report the long-term results of RTOG 9801, a randomized trial investigating the ability of amifostine, a radioprotector, to reduce chemoradiation-induced esophagitis.
Methods: Patients with stages II and IIIA/B non-small-cell lung cancer received induction paclitaxel 225 mg/m2 intravenously (IV) and carboplatin area under the curve (AUC) 6 both days 1 and 22, followed by concurrent weekly paclitaxel (50 mg/m2) and carboplatin (AUC 2), with hyperfractionated radiation therapy (69.6 Gy at 1.2 Gy BID). Patients were randomly assigned to amifostine (AM) 500 mg IV four times per week or no-AM during chemoradiotherapy. Stratification factors included age (<70 vs. ≥70 years), stage and performance status.
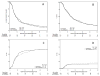
Results: 243 patients (pts) were enrolled; 120 received AM, 123 received no-AM. Two pts on each arm were found ineligible. Overall, 85% of patients were ≤70 years; 75% had a KPS ≥90. 34% had squamous histology. With median follow-up of 96.3 months (for patients still alive), overall survival was identical (hazard ratio 1.03 (0.79-1.34), NS): five-year survival 17% in both arms. The incidence of late grade 3-5 toxicities was 16% in the AM arm and 19% in the control arm (hazard ratio 1.24 (0.66-2.32), NS). There was no significant difference between the arms regarding overall survival, disease-free survival or long-term toxicity.
Conclusion: The chemoradiation regimen of carboplatin and paclitaxel produced long-term results in the multi-institutional setting comparable to other regimens. Amifostine did not appear to compromise survival. As done in RTOG 9801, more consistent reporting of long term toxicity is needed for comparison of different chemoradiation regimens.
Trial registration: ClinicalTrials.gov NCT00003313.
Copyright © 2013 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
Past advisory activity with Medimmune and BMS [CJ Langer].
None of the other co-authors have conflicts of interest to declare.
Figures
References
-
- Auperin A, Le Pechoux C, Rolland E, Curran WJ, Furuse K, Fournel P, Belderbos J, Clamon G, Ulutin HC, Paulus R, Yamanaka T, Bozonnat MC, Uitterhoeve A, Wang X, Stewart L, Arriagada R, Burdett S, Pignon JP. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–2190. - PubMed
-
- Komaki R, Scott CB, Sause WT, Johnson DH, Taylor SGt, Lee JS, Emami B, Byhardt RW, Curran WJ, Jr, Dar AR, Cox JD. Induction cisplatin/vinblastine and irradiation vs. irradiation in unresectable squamous cell lung cancer: failure patterns by cell type in RTOG 88-08/ECOG 4588. Radiation Therapy Oncology Group. Eastern Cooperative Oncology Group. Int J Radiat Oncol Biol Phys. 1997;39:537–544. - PubMed
-
- Rowell NP, O’rourke NP. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev. 2004:CD002140. - PubMed
-
- Curran WJ, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, Movsas B, Wasserman T, Rosenthal SA, Gore E, Machtay M, Sause W, Cox JD. Sequential vs Concurrent Chemoradiation for Stage III Non Small Cell Lung Cancer: Randomized Phase III Trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452–1460. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

