Epigenetic reprogramming of human embryonic stem cells into skeletal muscle cells and generation of contractile myospheres
- PMID: 23478022
- PMCID: PMC3625045
- DOI: 10.1016/j.celrep.2013.02.012
Epigenetic reprogramming of human embryonic stem cells into skeletal muscle cells and generation of contractile myospheres
Abstract
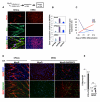
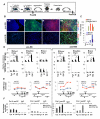
Direct generation of a homogeneous population of skeletal myoblasts from human embryonic stem cells (hESCs) and formation of three-dimensional contractile structures for disease modeling in vitro are current challenges in regenerative medicine. Previous studies reported on the generation of myoblasts from ESC-derived embryoid bodies (EB), but not from undifferentiated ESCs, indicating the requirement for mesodermal transition to promote skeletal myogenesis. Here, we show that selective absence of the SWI/SNF component BAF60C (encoded by SMARCD3) confers on hESCs resistance to MyoD-mediated activation of skeletal myogenesis. Forced expression of BAF60C enables MyoD to directly activate skeletal myogenesis in hESCs by instructing MyoD positioning and allowing chromatin remodeling at target genes. BAF60C/MyoD-expressing hESCs are epigenetically committed myogenic progenitors, which bypass the mesodermal requirement and, when cultured as floating clusters, give rise to contractile three-dimensional myospheres composed of skeletal myotubes. These results identify BAF60C as a key epigenetic determinant of hESC commitment to the myogenic lineage and establish the molecular basis for the generation of hESC-derived myospheres exploitable for "disease in a dish" models of muscular physiology and dysfunction.
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Azuara V, Perry P, Sauer S, Spivakov M, Jorgensen HF, John RM, Gouti M, Casanova M, Warnes G, Merkenschlager M, et al. Chromatin signatures of pluripotent cell lines. Nat Cell Biol. 2006;8:532–538. - PubMed
-
- Barberi T, Bradbury M, Dincer Z, Panagiotakos G, Socci ND, Studer L. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med. 2007;13:642–648. - PubMed
-
- Berkes CA, Bergstrom DA, Penn BH, Seaver KJ, Knoepfler PS, Tapscott SJ. Pbx marks genes for activation by MyoD indicating a role for a homeodomain protein in establishing myogenic potential. Mol Cell. 2004;14:465–477. - PubMed
-
- Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, Levine SS, Wernig M, Tajonar A, Ray MK, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–353. - PubMed
-
- Buckingham M, Relaix F. The role of Pax genes in the development of tissues and organs: Pax3 and Pax7 regulate muscle progenitor cell functions. Annu Rev Cell Dev Biol. 2007;23:645–673. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

