Plasma glutamate-modulated interaction of A2AR and mGluR5 on BMDCs aggravates traumatic brain injury-induced acute lung injury
- PMID: 23478188
- PMCID: PMC3620361
- DOI: 10.1084/jem.20122196
Plasma glutamate-modulated interaction of A2AR and mGluR5 on BMDCs aggravates traumatic brain injury-induced acute lung injury
Abstract
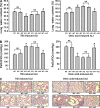
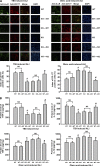
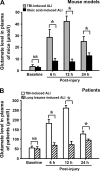
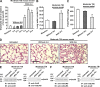
The bone marrow-derived cell (BMDC)-associated inflammatory response plays a key role in the development of acute lung injury (ALI). Activation of adenosine A2A receptor (A2AR) is generally considered to be antiinflammatory, inhibiting BMDC activities to protect against ALI. However, in the present study, we found that in a mouse model of neurogenic ALI induced by severe traumatic brain injury (TBI), BMDC A2AR exerted a proinflammatory effect, aggravating lung damage. This is in contrast to the antiinflammatory effect observed in the mouse oleic acid-induced ALI model (a nonneurogenic ALI model.) Moreover, the A2AR agonist CGS21680 aggravated, whereas the antagonist ZM241385 attenuated, the severe TBI-induced lung inflammatory damage in mice. Further investigation of white blood cells isolated from patients or mouse TBI models and of cultured human or mouse neutrophils demonstrated that elevated plasma glutamate after severe TBI induced interaction between A2AR and the metabotropic glutamate receptor 5 (mGluR5) to increase phospholipase C-protein kinase C signaling, which mediated the proinflammatory effect of A2AR. These results are in striking contrast to the well-known antiinflammatory and protective role of A2AR in nonneurogenic ALI and indicate different therapeutic strategies should be used for nonneurogenic and neurogenic ALI treatment when targeting A2AR.
Figures



Similar articles
-
PKC Mediates LPS-Induced IL-1β Expression and Participates in the Pro-inflammatory Effect of A2AR Under High Glutamate Concentrations in Mouse Microglia.Neurochem Res. 2019 Dec;44(12):2755-2764. doi: 10.1007/s11064-019-02895-1. Epub 2019 Oct 24. Neurochem Res. 2019. PMID: 31650360
-
Reduction in Blood Glutamate Levels Combined With the Genetic Inactivation of A2AR Significantly Alleviate Traumatic Brain Injury-Induced Acute Lung Injury.Shock. 2019 Apr;51(4):502-510. doi: 10.1097/SHK.0000000000001170. Shock. 2019. PMID: 29688987
-
Adenosine A2A receptor inactivation alleviates early-onset cognitive dysfunction after traumatic brain injury involving an inhibition of tau hyperphosphorylation.Transl Psychiatry. 2017 May 9;7(5):e1123. doi: 10.1038/tp.2017.98. Transl Psychiatry. 2017. PMID: 28485728 Free PMC article.
-
The coming together of allosteric and phosphorylation mechanisms in the molecular integration of A2A heteroreceptor complexes in the dorsal and ventral striatal-pallidal GABA neurons.Pharmacol Rep. 2021 Aug;73(4):1096-1108. doi: 10.1007/s43440-021-00314-3. Epub 2021 Aug 24. Pharmacol Rep. 2021. PMID: 34426901 Free PMC article. Review.
-
Recent advances in the role of the adenosinergic system in coronary artery disease.Cardiovasc Res. 2021 Apr 23;117(5):1284-1294. doi: 10.1093/cvr/cvaa275. Cardiovasc Res. 2021. PMID: 32991685 Review.
Cited by
-
PKC Mediates LPS-Induced IL-1β Expression and Participates in the Pro-inflammatory Effect of A2AR Under High Glutamate Concentrations in Mouse Microglia.Neurochem Res. 2019 Dec;44(12):2755-2764. doi: 10.1007/s11064-019-02895-1. Epub 2019 Oct 24. Neurochem Res. 2019. PMID: 31650360
-
The Dual Role of A2aR in Neuroinflammation: Modulating Microglial Polarization in White Matter Lesions.eNeuro. 2025 Mar 14;12(3):ENEURO.0579-24.2025. doi: 10.1523/ENEURO.0579-24.2025. Print 2025 Mar. eNeuro. 2025. PMID: 39947902 Free PMC article.
-
A2A R inhibition in alleviating spatial recognition memory impairment after TBI is associated with improvement in autophagic flux in RSC.J Cell Mol Med. 2020 Jun;24(12):7000-7014. doi: 10.1111/jcmm.15361. Epub 2020 May 12. J Cell Mol Med. 2020. PMID: 32394486 Free PMC article.
-
HMGB1 mediates cognitive impairment caused by the NLRP3 inflammasome in the late stage of traumatic brain injury.J Neuroinflammation. 2021 Oct 19;18(1):241. doi: 10.1186/s12974-021-02274-0. J Neuroinflammation. 2021. PMID: 34666797 Free PMC article.
-
Metabotropic glutamate receptor 5 deficiency inhibits neutrophil infiltration after traumatic brain injury in mice.Sci Rep. 2017 Aug 30;7(1):9998. doi: 10.1038/s41598-017-10201-8. Sci Rep. 2017. PMID: 28855570 Free PMC article.
References
-
- Abraham E., Carmody A., Shenkar R., Arcaroli J. 2000. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 279:L1137–L1145 - PubMed
-
- Aliprandi A., Longoni M., Stanzani L., Tremolizzo L., Vaccaro M., Begni B., Galimberti G., Garofolo R., Ferrarese C. 2005. Increased plasma glutamate in stroke patients might be linked to altered platelet release and uptake. J. Cereb. Blood Flow Metab. 25:513–519 10.1038/sj.jcbfm.9600039 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

