Rab12 regulates mTORC1 activity and autophagy through controlling the degradation of amino-acid transporter PAT4
- PMID: 23478338
- PMCID: PMC3642374
- DOI: 10.1038/embor.2013.32
Rab12 regulates mTORC1 activity and autophagy through controlling the degradation of amino-acid transporter PAT4
Abstract
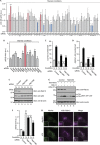
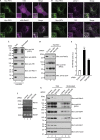
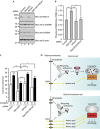
Autophagy is an evolutionarily conserved catabolic mechanism that targets intracellular molecules and damaged organelles to lysosomes. Autophagy is achieved by a series of membrane trafficking events, but their regulatory mechanisms are poorly understood. Here, we report small GTPase Rab12 as a new type of autophagic regulator that controls the degradation of an amino-acid transporter. Knockdown of Rab12 results in inhibition of autophagy and in increased activity of mTORC1 (mammalian/mechanistic target of rapamycin complex 1), an upstream regulator of autophagy. We also found that Rab12 promotes constitutive degradation of PAT4 (proton-coupled amino-acid transporter 4), whose accumulation in Rab12-knockdown cells modulates mTORC1 activity and autophagy. Our findings reveal a new mechanism of regulation of mTORC1 signalling and autophagy, that is, quality control of PAT4 by Rab12.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Comment in
-
On the Rab again--the PATh to mTORC1 activation.EMBO Rep. 2013 May;14(5):398-9. doi: 10.1038/embor.2013.48. Epub 2013 Apr 19. EMBO Rep. 2013. PMID: 23598518 Free PMC article. No abstract available.
References
-
- Mizushima N, Komatsu M (2011) Autophagy: renovation of cells and tissues. Cell 147: 728–741 - PubMed
-
- Mizushima N (2007) Autophagy: process and function. Genes Dev 21: 2861–2873 - PubMed
-
- Stenmark H (2009) Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10: 513–525 - PubMed
-
- Fader CM, Sánchez D, Furlán M, Colombo MI (2008) Induction of autophagy promotes fusion of multivesicular bodies with autophagic vacuoles in k562 cells. Traffic 9: 230–250 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

