CRL1-FBXO11 promotes Cdt2 ubiquitylation and degradation and regulates Pr-Set7/Set8-mediated cellular migration
- PMID: 23478445
- PMCID: PMC3615078
- DOI: 10.1016/j.molcel.2013.02.003
CRL1-FBXO11 promotes Cdt2 ubiquitylation and degradation and regulates Pr-Set7/Set8-mediated cellular migration
Abstract
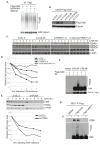
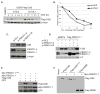
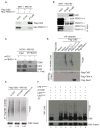
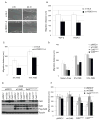
The Cul4-Cdt2 (CRL4(Cdt2)) E3 ubiquitin ligase is a master regulator of cell-cycle progression and genome stability. Despite its central role in the degradation of many cell-cycle regulators, e.g., Cdt1, p21, and Pr-Set7/Set8, little is known about the regulation of its activity. We report that Cdt2 is autoubiquitylated by the CRL4A E3 ubiquitin ligase. Cdt2 is additionally polyubiquitylated and degraded by Cul1-FBXO11 (CRL1(FBXO11)). CRL1(FBXO11)-mediated degradation of Cdt2 stabilizes p21 and Set8, and this is important during the response to TGF-β, with the Set8 induction being important for turning off the activation of Smad2. The migration of epithelial cells is also stimulated by CRL1(FBXO11)-mediated downregulation of Cdt2 and the consequent stabilization of Set8. This is an interesting example of cross-regulation between specific Cullin 4 and Cullin 1 E3 ubiquitin ligases and highlights the role of ubiquitylation in regulating cellular responses to TGF-β and the migration of epithelial cells.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures



References
-
- Angers S, Thorpe CJ, Biechele TL, Goldenberg SJ, Zheng N, MacCoss MJ, Moon RT. The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt-beta-catenin pathway by targeting Dishevelled for degradation. Nat Cell Biol. 2006;8:348–357. - PubMed
-
- Baraniskin A, Birkenkamp-Demtroder K, Maghnouj A, Zollner H, Munding J, Klein-Scory S, Reinacher-Schick A, Schwarte-Waldhoff I, Schmiegel W, Hahn SA. MiR-30a-5p suppresses tumor growth in colon carcinoma by targeting DTL. Carcinogenesis. 2012;33:732–739. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

