Clopidogrel and warfarin pharmacogenetic tests: what is the evidence for use in clinical practice?
- PMID: 23478884
- PMCID: PMC3731766
- DOI: 10.1097/HCO.0b013e32835f0bbc
Clopidogrel and warfarin pharmacogenetic tests: what is the evidence for use in clinical practice?
Abstract
Purpose of review: To review the most promising genetic markers associated with the variability in the safety or efficacy of warfarin and clopidogrel and highlight the verification and validation initiatives for translating clopidogrel and warfarin pharmacogenetic tests to clinical practice.
Recent findings: Rapid advances in pharmacogenetics, continuous decrease in genotyping cost, development of point-of-care devices and the newly established clinical genotyping programs at several institutions hold the promise of individualizing clopidogrel and warfarin based on genotype. Guidelines have been established to assist clinicians in prescribing clopidogrel or warfarin dose based on genotype. However, the clinical utility of clopidogrel and warfarin is still limited. Accordingly, large randomized clinical trials are underway to define the role of clopidogrel and warfarin pharmacogenetics in clinical practice.
Summary: Pharmacogenetics has offered compelling evidence toward the individualization of clopidogrel and warfarin therapies. The rapid advances in technology make the clinical implementation of clopidogrel and warfarin pharmacogenetics possible. The clinical genotyping programs and the ongoing clinical trials will help in overcoming some of the barriers facing the clinical implementation of clopidogrel and warfarin pharmacogenetics.
Conflict of interest statement
Figures

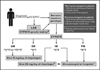
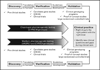
References
-
- World Health Organization. Global status report on noncommunicable diseases 2010. Geneva, Switzerland: WHO; 2010.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

