Extracellular DNA shields against aminoglycosides in Pseudomonas aeruginosa biofilms
- PMID: 23478967
- PMCID: PMC3632962
- DOI: 10.1128/AAC.00001-13
Extracellular DNA shields against aminoglycosides in Pseudomonas aeruginosa biofilms
Abstract
Within recent years, it has been established that extracellular DNA is a key constituent of the matrix of microbial biofilms. In addition, it has recently been demonstrated that DNA binds positively charged antimicrobials such as aminoglycosides and antimicrobial peptides. In the present study, we provide evidence that extracellular DNA shields against aminoglycosides in Pseudomonas aeruginosa biofilms. We show that exogenously supplemented DNA integrates into P. aeruginosa biofilms and increases their tolerance toward aminoglycosides. We provide evidence that biofilms formed by a DNA release-deficient P. aeruginosa quorum-sensing mutant are more susceptible to aminoglycoside treatment than wild-type biofilms but become rescued from the detrimental action of aminoglycosides upon supplementation with exogenous DNA. Furthermore, we demonstrate that exposure to lysed polymorphonuclear leukocytes, which are thought to be a source of extracellular DNA at sites of infections, increases the tolerance of P. aeruginosa biofilms toward aminoglycosides. Although biofilm-associated aminoglycoside tolerance recently has been linked to extracellular DNA-mediated activation of the pmr genes, we demonstrate that the aminoglycoside tolerance mediated by the presence of extracellular DNA is not caused by activation of the pmr genes in our P. aeruginosa biofilms but rather by a protective shield effect of the extracellular DNA.
Figures
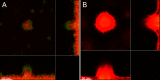
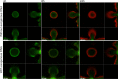
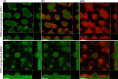
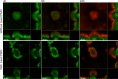
References
-
- Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322 - PubMed
-
- Ciofu O, Tolker-Nielsen T. 2010. Antibiotic tolerance and resistance in biofilms, p 215–230 In Bjarnsholt T, Jensen PØ, Moser C, Høiby N. (ed), Biofilm infections. Springer Publishing Company, New York, NY
-
- Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. 2002. Extracellular DNA required for bacterial biofilm formation. Science 295:1487. - PubMed
-
- Friedman L, Kolter R. 2003. Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Mol. Microbiol. 51:675–690 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

