Monocyte ADAM17 promotes diapedesis during transendothelial migration: identification of steps and substrates targeted by metalloproteinases
- PMID: 23479224
- PMCID: PMC3622190
- DOI: 10.4049/jimmunol.1300046
Monocyte ADAM17 promotes diapedesis during transendothelial migration: identification of steps and substrates targeted by metalloproteinases
Abstract
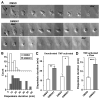
Despite expanded definition of the leukocyte adhesion cascade and mechanisms underlying individual steps, very little is known about regulatory mechanisms controlling sequential shifts between steps. We tested the hypothesis that metalloproteinases provide a mechanism to rapidly transition monocytes between different steps. Our study identifies diapedesis as a step targeted by metalloproteinase activity. Time-lapse video microscopy shows that the presence of a metalloproteinase inhibitor results in a doubling of the time required for human monocytes to complete diapedesis on unactivated or inflamed human endothelium, under both static and physiological-flow conditions. Thus, diapedesis is promoted by metalloproteinase activity. In contrast, neither adhesion of monocytes nor their locomotion over the endothelium is altered by metalloproteinase inhibition. We further demonstrate that metalloproteinase inhibition significantly elevates monocyte cell surface levels of integrins CD11b/CD18 (Mac-1), specifically during transendothelial migration. Interestingly, such alterations are not detected for other endothelial- and monocyte-adhesion molecules that are presumed metalloproteinase substrates. Two major transmembrane metalloproteinases, a disintegrin and metalloproteinase (ADAM)17 and ADAM10, are identified as enzymes that control constitutive cleavage of Mac-1. We further establish that knockdown of monocyte ADAM17, but not endothelial ADAM10 or ADAM17 or monocyte ADAM10, reproduces the diapedesis delay observed with metalloproteinase inhibition. Therefore, we conclude that monocyte ADAM17 facilitates the completion of transendothelial migration by accelerating the rate of diapedesis. We propose that the progression of diapedesis may be regulated by spatial and temporal cleavage of Mac-1, which is triggered upon interaction with endothelium.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures
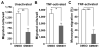
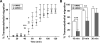



Similar articles
-
ADAM10-Mediated Cleavage of ICAM-1 Is Involved in Neutrophil Transendothelial Migration.Cells. 2021 Jan 25;10(2):232. doi: 10.3390/cells10020232. Cells. 2021. PMID: 33504031 Free PMC article.
-
Regulated release and functional modulation of junctional adhesion molecule A by disintegrin metalloproteinases.Blood. 2009 May 7;113(19):4799-809. doi: 10.1182/blood-2008-04-152330. Epub 2009 Mar 3. Blood. 2009. PMID: 19258599
-
Novel ex vivo culture method for human monocytes uses shear flow to prevent total loss of transendothelial diapedesis function.J Leukoc Biol. 2014 Jan;95(1):191-5. doi: 10.1189/jlb.0513272. Epub 2013 Sep 4. J Leukoc Biol. 2014. PMID: 24006509 Free PMC article.
-
Metalloproteinase inhibitors for the disintegrin-like metalloproteinases ADAM10 and ADAM17 that differentially block constitutive and phorbol ester-inducible shedding of cell surface molecules.Comb Chem High Throughput Screen. 2005 Mar;8(2):161-71. doi: 10.2174/1386207053258488. Comb Chem High Throughput Screen. 2005. PMID: 15777180 Review.
-
Transendothelial migration of monocytes: the underlying molecular mechanisms and consequences of HIV-1 infection.Curr HIV Res. 2005 Oct;3(4):303-17. doi: 10.2174/157016205774370401. Curr HIV Res. 2005. PMID: 16250878 Review.
Cited by
-
Neutrophil and Macrophage Cell Surface Colony-Stimulating Factor 1 Shed by ADAM17 Drives Mouse Macrophage Proliferation in Acute and Chronic Inflammation.Mol Cell Biol. 2018 Aug 15;38(17):e00103-18. doi: 10.1128/MCB.00103-18. Print 2018 Sep 1. Mol Cell Biol. 2018. PMID: 29891514 Free PMC article.
-
An Allosteric Shift in CD11c Affinity Activates a Proatherogenic State in Arrested Intermediate Monocytes.J Immunol. 2020 Nov 15;205(10):2806-2820. doi: 10.4049/jimmunol.2000485. Epub 2020 Oct 14. J Immunol. 2020. PMID: 33055281 Free PMC article.
-
ADAM17 Boosts Cholesterol Efflux and Downstream Effects of High-Density Lipoprotein on Inflammatory Pathways in Macrophages.Arterioscler Thromb Vasc Biol. 2021 Jun;41(6):1854-1873. doi: 10.1161/ATVBAHA.121.315145. Epub 2021 Apr 22. Arterioscler Thromb Vasc Biol. 2021. PMID: 33882688 Free PMC article.
-
ADAM10-Mediated Cleavage of ICAM-1 Is Involved in Neutrophil Transendothelial Migration.Cells. 2021 Jan 25;10(2):232. doi: 10.3390/cells10020232. Cells. 2021. PMID: 33504031 Free PMC article.
-
Survey of In Vitro Model Systems for Investigation of Key Cellular Processes Associated with Atherosclerosis.Methods Mol Biol. 2022;2419:39-56. doi: 10.1007/978-1-0716-1924-7_3. Methods Mol Biol. 2022. PMID: 35237957
References
-
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. - PubMed
-
- Petri B, Phillipson M, Kubes P. The physiology of leukocyte recruitment: an in vivo perspective. J Immunol. 2008;180:6439–6446. - PubMed
-
- Garton KJ, Gough PJ, Raines EW. Emerging roles for ectodomain shedding in the regulation of inflammatory responses. J Leukoc Biol. 2006;79:1105–1116. - PubMed
-
- Reiss K, Saftig P. The “a disintegrin and metalloprotease” (ADAM) family of sheddases: physiological and cellular functions. Semin Cell Dev Biol. 2009;20:126–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

