Hydrogen sulfide preconditions the db/db diabetic mouse heart against ischemia-reperfusion injury by activating Nrf2 signaling in an Erk-dependent manner
- PMID: 23479260
- PMCID: PMC3652093
- DOI: 10.1152/ajpheart.00796.2012
Hydrogen sulfide preconditions the db/db diabetic mouse heart against ischemia-reperfusion injury by activating Nrf2 signaling in an Erk-dependent manner
Abstract
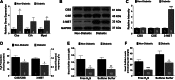
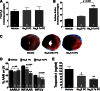
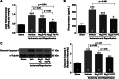
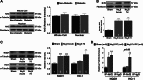
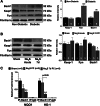
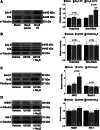
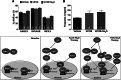
Hydrogen sulfide (H2S) therapy protects nondiabetic animals in various models of myocardial injury, including acute myocardial infarction and heart failure. Here, we sought to examine whether H2S therapy provides cardioprotection in the setting of type 2 diabetes. H2S therapy in the form of sodium sulfide (Na2S) beginning 24 h or 7 days before myocardial ischemia significantly decreased myocardial injury in db/db diabetic mice (12 wk of age). In an effort to evaluate the signaling mechanism responsible for the observed cardioprotection, we focused on the role of nuclear factor E2-related factor (Nrf2) signaling. Our results indicate that diabetes does not alter the ability of H2S to increase the nuclear localization of Nrf2, but does impair aspects of Nrf2 signaling. Specifically, the expression of NADPH quinine oxidoreductase 1 was increased after the acute treatment, whereas the expression of heme-oxygenase-1 (HO-1) was only increased after 7 days of treatment. This discrepancy was found to be the result of an increased nuclear expression of Bach1, a known repressor of HO-1 transcription, which blocked the binding of Nrf2 to the HO-1 promoter. Further analysis revealed that 7 days of Na2S treatment overcame this impairment by removing Bach1 from the nucleus in an Erk1/2-dependent manner. Our findings demonstrate for the first time that exogenous administration of Na2S attenuates myocardial ischemia-reperfusion injury in db/db mice, suggesting the potential therapeutic effects of H2S in treating a heart attack in the setting of type 2 diabetes.
Keywords: hydrogen sulfide; myocardial infarction; nuclear factor E2-related factor; type 2 diabetes.
Figures
References
-
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87: 840–844, 2000 - PubMed
-
- Calvert JW, Gundewar S, Jha S, Elrod JW, Lefer DJ. Hydrogen sulfide therapy attenuates left ventricular dysfunction and reduces mortality in a murine model of heart failure. Circulation 118: S441–S331, 2008
-
- Denizalti M, Bozkurt TE, Akpulat U, Sahin-Erdemli I, Abacioglu N. The vasorelaxant effect of hydrogen sulfide is enhanced in streptozotocin-induced diabetic rats. Naunyn Schmiedebergs Arch Pharmacol 383: 509–517, 2011 - PubMed
-
- El-Seweidy MM, Sadik NA, Shaker OG. Role of sulfurous mineral water and sodium hydrosulfide as potent inhibitors of fibrosis in the heart of diabetic rats. Arch Biochem Biophys 506: 48–57, 2011 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

