Anti-HER3 domain 1 and 3 antibodies reduce tumor growth by hindering HER2/HER3 dimerization and AKT-induced MDM2, XIAP, and FoxO1 phosphorylation
- PMID: 23479511
- PMCID: PMC3593156
- DOI: 10.1593/neo.121960
Anti-HER3 domain 1 and 3 antibodies reduce tumor growth by hindering HER2/HER3 dimerization and AKT-induced MDM2, XIAP, and FoxO1 phosphorylation
Abstract
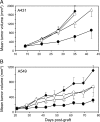
Blockade of the human epidermal growth factor receptor 3 (HER3) and of the downstream phosphatidylinositide 3-kinase (PI3K)/AKT pathway is a prerequisite for overcoming drug resistance and to develop novel treatments for cancers that are not eligible for the currently approved targeted therapies. To this end, we generated specific antibodies (Abs) against domain 1 (D1) and domain 3 (D3) of HER3 that recognize epitopes that do not overlap with the neuregulin-binding site. The fully human H4B-121 Ab and the mouse monoclonal Abs 16D3-C1 and 9F7-F11 inhibited tumor growth in nude mice xenografted with epidermoid, pancreatic, or triple-negative breast cancer cells. The combination of one anti-HER3 Ab and trastuzumab improved tumor growth inhibition in mice xenografted with HER2(low) cancer cell lines, for which trastuzumab alone shows no or moderate efficiency. Ab-induced disruption of tumor growth was associated with G1 cell cycle arrest, proliferation inhibition, and apoptosis of cancer cells. Anti-HER3 Abs blocked HER2/HER3 heterodimerization and HER3 phosphorylation at the cell membrane, leading to inhibition of phosphorylation of the downstream AKT targets murine double minute 2, X-linked inhibitor of apoptosis, and forkhead box O1. This study demonstrates that anti-HER3 D1 and D3 Abs could represent a new option for immunotherapy of pancreatic and triple-negative breast cancers.
Figures







Similar articles
-
Combination of antibody that inhibits ligand-independent HER3 dimerization and a p110α inhibitor potently blocks PI3K signaling and growth of HER2+ breast cancers.Cancer Res. 2013 Oct 1;73(19):6013-23. doi: 10.1158/0008-5472.CAN-13-1191. Epub 2013 Aug 5. Cancer Res. 2013. PMID: 23918797 Free PMC article.
-
An antibody that locks HER3 in the inactive conformation inhibits tumor growth driven by HER2 or neuregulin.Cancer Res. 2013 Oct 1;73(19):6024-35. doi: 10.1158/0008-5472.CAN-13-1198. Epub 2013 Aug 8. Cancer Res. 2013. PMID: 23928993 Free PMC article.
-
ERBB3 (HER3) is a key sensor in the regulation of ERBB-mediated signaling in both low and high ERBB2 (HER2) expressing cancer cells.Cancer Med. 2012 Aug;1(1):28-38. doi: 10.1002/cam4.10. Epub 2012 Jul 15. Cancer Med. 2012. PMID: 23342251 Free PMC article.
-
Biology of HER2 and its importance in breast cancer.Oncology. 2001;61 Suppl 2:1-13. doi: 10.1159/000055396. Oncology. 2001. PMID: 11694782 Review.
-
HER3: Toward the Prognostic Significance, Therapeutic Potential, Current Challenges, and Future Therapeutics in Different Types of Cancer.Cells. 2023 Oct 25;12(21):2517. doi: 10.3390/cells12212517. Cells. 2023. PMID: 37947595 Free PMC article. Review.
Cited by
-
Xiao-Ai-Ping, a TCM Injection, Enhances the Antigrowth Effects of Cisplatin on Lewis Lung Cancer Cells through Promoting the Infiltration and Function of CD8(+) T Lymphocytes.Evid Based Complement Alternat Med. 2013;2013:879512. doi: 10.1155/2013/879512. Epub 2013 Jul 17. Evid Based Complement Alternat Med. 2013. PMID: 23956781 Free PMC article.
-
Thirty Years of HER3: From Basic Biology to Therapeutic Interventions.Clin Cancer Res. 2021 Jul 1;27(13):3528-3539. doi: 10.1158/1078-0432.CCR-20-4465. Epub 2021 Feb 19. Clin Cancer Res. 2021. PMID: 33608318 Free PMC article. Review.
-
Targeting HER3 using mono- and bispecific antibodies or alternative scaffolds.MAbs. 2016 Oct;8(7):1195-1209. doi: 10.1080/19420862.2016.1212147. Epub 2016 Aug 17. MAbs. 2016. PMID: 27532938 Free PMC article. Review.
-
Novel therapeutic targets for pancreatic cancer.World J Gastroenterol. 2014 Aug 21;20(31):10825-44. doi: 10.3748/wjg.v20.i31.10825. World J Gastroenterol. 2014. PMID: 25152585 Free PMC article. Review.
-
HER3 as biomarker and therapeutic target in pancreatic cancer: new insights in pertuzumab therapy in preclinical models.Oncotarget. 2014 Aug 30;5(16):7138-48. doi: 10.18632/oncotarget.2231. Oncotarget. 2014. PMID: 25216528 Free PMC article.
References
-
- Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer. 2012;12:553–563. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
