Efficient generation of stable bispecific IgG1 by controlled Fab-arm exchange
- PMID: 23479652
- PMCID: PMC3612680
- DOI: 10.1073/pnas.1220145110
Efficient generation of stable bispecific IgG1 by controlled Fab-arm exchange
Abstract
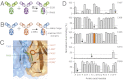
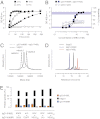
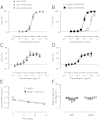
The promise of bispecific antibodies (bsAbs) to yield more effective therapeutics is well recognized; however, the generation of bsAbs in a practical and cost-effective manner has been a formidable challenge. Here we present a technology for the efficient generation of bsAbs with normal IgG structures that is amenable to both antibody drug discovery and development. The process involves separate expression of two parental antibodies, each containing single matched point mutations in the CH3 domains. The parental antibodies are mixed and subjected to controlled reducing conditions in vitro that separate the antibodies into HL half-molecules and allow reassembly and reoxidation to form highly pure bsAbs. The technology is compatible with standard large-scale antibody manufacturing and ensures bsAbs with Fc-mediated effector functions and in vivo stability typical of IgG1 antibodies. Proof-of-concept studies with HER2×CD3 (T-cell recruitment) and HER2×HER2 (dual epitope targeting) bsAbs demonstrate superior in vivo activity compared with parental antibody pairs.
Conflict of interest statement
Conflict of interest statement: The authors have stock and/or warrants in Genmab.
Figures


References
-
- Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol. 2010;10(5):301–316. - PubMed
-
- Carter P. Bispecific human IgG by design. J Immunol Methods. 2001;248(1–2):7–15. - PubMed
-
- Chames P, Baty D. Bispecific antibodies for cancer therapy. Curr Opin Drug Discov Devel. 2009;12(2):276–283. - PubMed
-
- Kufer P, Lutterbüse R, Baeuerle PA. A revival of bispecific antibodies. Trends Biotechnol. 2004;22(5):238–244. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

