Rer1p maintains ciliary length and signaling by regulating γ-secretase activity and Foxj1a levels
- PMID: 23479743
- PMCID: PMC3601348
- DOI: 10.1083/jcb.201208175
Rer1p maintains ciliary length and signaling by regulating γ-secretase activity and Foxj1a levels
Abstract
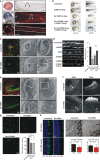
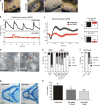
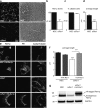
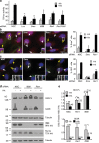
Cilia project from the surface of most vertebrate cells and are important for several physiological and developmental processes. Ciliary defects are linked to a variety of human diseases, named ciliopathies, underscoring the importance of understanding signaling pathways involved in cilia formation and maintenance. In this paper, we identified Rer1p as the first endoplasmic reticulum/cis-Golgi-localized membrane protein involved in ciliogenesis. Rer1p, a protein quality control receptor, was highly expressed in zebrafish ciliated organs and regulated ciliary structure and function. Both in zebrafish and mammalian cells, loss of Rer1p resulted in the shortening of cilium and impairment of its motile or sensory function, which was reflected by hearing, vision, and left-right asymmetry defects as well as decreased Hedgehog signaling. We further demonstrate that Rer1p depletion reduced ciliary length and function by increasing γ-secretase complex assembly and activity and, consequently, enhancing Notch signaling as well as reducing Foxj1a expression.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

