Early tumor shrinkage in metastatic colorectal cancer: retrospective analysis from an irinotecan-based randomized first-line trial
- PMID: 23480146
- PMCID: PMC7657245
- DOI: 10.1111/cas.12148
Early tumor shrinkage in metastatic colorectal cancer: retrospective analysis from an irinotecan-based randomized first-line trial
Abstract
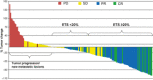
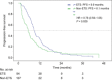
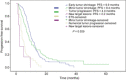
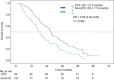
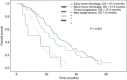
Early tumor shrinkage (ETS) has been highlighted as a favorable prognostic factor related to progression-free survival (PFS) and overall survival (OS) in cytotoxic treatment of metastatic colorectal cancer. Data from a randomized phase III study comparing infusional 5-fluorouracil plus irinotecan (FUFIRI) versus irinotecan plus oxaliplatin (mIROX) were evaluated. Patient groups were analyzed according to the relative change in maximum tumor diameter between baseline and after 7 weeks of treatment. The ETS cohort was defined as a decrease of ≥20%. Additionally, the non-ETS cohort was subdivided into "minor shrinkage" (0-19%), "tumor progression" (any increase) and development of "new metastatic lesions". Progression-free survival and OS were estimated in all patient subgroups. Assessment of ETS was possible in 201 patients. Early tumor shrinkage was observed in 47% (94/201) and non-ETS in 53% (107/201) of patients. Patients with ETS had a more favorable outcome with regard to PFS (9.9 months vs 6.1 months, P = 0.029) and OS (27.5 months vs 17.8 months, P = 0.002). In the non-ETS subgroups, patients with "minor shrinkage" (PFS 8.4 months, OS 21.6 months) showed a markedly better outcome than patients with "early tumor progression" (PFS 4.0 months, OS 15.3 months) or with "new metastatic lesions (PFS 2.2 months, OS 7.6 months). In conclusion, ETS assessment offers accelerated response evaluation when compared to RECIST. In patients treated with chemotherapy alone, ETS ≥20% is associated with excellent outcome. Non-ETS is a heterogeneous subgroup where patients with minor shrinkage clearly benefit from treatment, and patients with early progression or development of new lesions have an unfavorable prognosis.
© 2013 Japanese Cancer Association.
Figures
References
-
- Eisenhauer EA, Therasse P, Bogaerts J et al New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. - PubMed
-
- Piessevaux H, Buyse M, De Roock W et al Radiological tumor size decrease at week 6 is a potent predictor of outcome in chemorefractory metastatic colorectal cancer treated with cetuximab (BOND trial). Ann Oncol 2009; 20: 1375–82. - PubMed
-
- Piessevaux H, Schlichting M, Heeger S, Van Cutsem EST. Early tumor shrinkage for the prediction of efficacy of cetuximab in metastatic colorectal cancer (mCRC): analysis from the CRYSTAL study. Abstracts of the 35th European Society for Medical Oncology Conference. October 8–12, 2010. Milan, Italy. Ann Oncol. 2010; 21(Suppl 8): viii 189–viii 224.
-
- Piessevaux H, Van Cutsem E, Bokemeyer C, Schlichting M, Heeger S, Tejpar S. Early tumor shrinkage and clinical outcome in metastatic colorectal cancer: Analysis of predictive utility in the CRYSTAL and OPUS studies. Oral presentation at the 10th Annual Meeting of the Japanese Society of Medical Oncology (JSMO), Osaka July 28th. 2012.
-
- Glimelius B, Sorbye H, Balteskard L et al A randomized phase III multicenter trial comparing irinotecan in combination with the Nordic bolus 5‐FU and folinic acid schedule or the bolus/infused de Gramont schedule (Lv5FU2) in patients with metastatic colorectal cancer. Ann Oncol 2008; 19: 909–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

