Red blood cells stored for increasing periods produce progressive impairments in nitric oxide-mediated vasodilation
- PMID: 23480490
- PMCID: PMC4140194
- DOI: 10.1111/trf.12111
Red blood cells stored for increasing periods produce progressive impairments in nitric oxide-mediated vasodilation
Abstract
Background: Clinical outcomes in transfused patients may be affected by the duration of blood storage, possibly due to red blood cell (RBC)-mediated disruption of nitric oxide (NO) signaling, a key regulator of vascular tone and blood flow.
Study design and methods: AS-1 RBC units stored up to 42 days were sampled at selected storage times. Samples were added to aortic rings ex vivo, a system where NO-mediated vasodilation could be experimentally controlled.
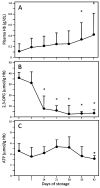
Results: RBC units showed storage-dependent changes in plasma hemoglobin (Hb), RBC 2,3-diphosphoglycerate acid, and RBC adenosine triphosphate conforming to expected profiles. When freshly collected (Day 0) blood was added to rat aortic rings, methacholine (MCh) stimulated substantial NO-mediated vasodilation. In contrast, MCh produced no vasodilation in the presence of blood stored for 42 days. Surprisingly, the vasoinhibitory effects of stored RBCs were almost totally mediated by RBCs themselves: removal of the supernatant did not attenuate the inhibitory effects, while addition of supernatant alone to the aortic rings only minimally inhibited MCh-stimulated relaxation. Stored RBCs did not inhibit vasodilation by a direct NO donor, demonstrating that the RBC-mediated vasoinhibitory mechanism did not work by NO scavenging.
Conclusions: These studies have revealed a previously unrecognized vasoinhibitory activity of stored RBCs, which is more potent than the described effects of free Hb and works through a different mechanism that does not involve NO scavenging but may function by reducing endothelial NO production. Through this novel mechanism, transfusion of small volumes of stored blood may be able to disrupt physiologic vasodilatory responses and thereby possibly cause adverse clinical outcomes.
© 2013 American Association of Blood Banks.
Figures
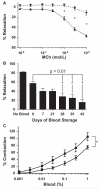
 , 1% final concentration), but not Day 42 saRBCs (
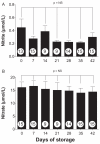
, 1% final concentration), but not Day 42 saRBCs ( , *p < 0.001; n = 14-19 experiments per data point). (B) Time course analysis shows that blood stored 28 days or longer significantly inhibits vasodilation compared to Day 0 saRBCs (* p < 0.01; n = 62 [no blood], n = 10-19 [data points with added blood]). (C) Day 42 saRBCs (
, *p < 0.001; n = 14-19 experiments per data point). (B) Time course analysis shows that blood stored 28 days or longer significantly inhibits vasodilation compared to Day 0 saRBCs (* p < 0.01; n = 62 [no blood], n = 10-19 [data points with added blood]). (C) Day 42 saRBCs ( ) were more effective than Day 0 samples (
) were more effective than Day 0 samples ( ) at stimulating vasoconstriction when added to prerelaxed vessels (*p < 0.01 for two curves by two-way analysis of variance; n = 6 samples per data point).
) at stimulating vasoconstriction when added to prerelaxed vessels (*p < 0.01 for two curves by two-way analysis of variance; n = 6 samples per data point).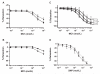
 ), or left unmanipulated (
), or left unmanipulated ( ), and then added to aortic rings before MCh. Removal of supernatant by washing did not significantly affect the vasoinhibition (p > 0.05; n = 5-11 samples per data point). (C) Compared to the no supernatant added control (○), addition of Day 0 saRBC supernatant (■, n = 6) did not inhibit MCh-stimulated vasodilation (p > 0.05; NS). However, Day 42 supernatant (▲) did cause a small but significant inhibition of vasodilation (*p < 0.01) at the highest four MCh concentrations when compared to no supernatant, but not when compared to Day 0 supernatant (p > 0.05; NS). These results suggest that saRBC supernatant accounts for only a small fraction of the vasoinhibitory activity of unmanipulated saRBC samples. (D) In contrast to the inhibitory activity of saRBCs on endothelium-dependent MCh-stimulated vasodilation, saRBCs had no effect on vasodilation induced by the endothelium-independent NO-donor SNP (p > 0.05; n = 4). (
), and then added to aortic rings before MCh. Removal of supernatant by washing did not significantly affect the vasoinhibition (p > 0.05; n = 5-11 samples per data point). (C) Compared to the no supernatant added control (○), addition of Day 0 saRBC supernatant (■, n = 6) did not inhibit MCh-stimulated vasodilation (p > 0.05; NS). However, Day 42 supernatant (▲) did cause a small but significant inhibition of vasodilation (*p < 0.01) at the highest four MCh concentrations when compared to no supernatant, but not when compared to Day 0 supernatant (p > 0.05; NS). These results suggest that saRBC supernatant accounts for only a small fraction of the vasoinhibitory activity of unmanipulated saRBC samples. (D) In contrast to the inhibitory activity of saRBCs on endothelium-dependent MCh-stimulated vasodilation, saRBCs had no effect on vasodilation induced by the endothelium-independent NO-donor SNP (p > 0.05; n = 4). ( ) Day 0; (
) Day 0; ( ) Day 42.
) Day 42.Comment in
-
Stored red blood cells impair vascular function in vivo.Transfusion. 2014 Jan;54(1):255. doi: 10.1111/trf.12436. Transfusion. 2014. PMID: 24405307 No abstract available.
References
-
- Wier LM, Pfuntner A, Maeda J, Stranges E, Ryan K, Jagadish P, Collins Sharp B, Elixhauser A. Health Care Cost and Utilization Project (HCUP) facts and figures, statistics on hospital-based care in the United States. Exhibit 3.1. Most frequent all-listed procedures. 2009 [cited 2013 Jan 14]. Available from: URL: http://www.hcup-us.ahrq.gov/reports/factsandfigures/2009/exhibit3_1.jsp.
-
- Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48:1053–60. - PubMed
-
- Zimrin AB, Hess JR. Current issues relating to the transfusion of stored red blood cells. Vox Sang. 2009;96:93–103. - PubMed
-
- Hovav T, Yedgar S, Manny N, Barshtein G. Alteration of red cell aggregability and shape during blood storage. Transfusion. 1999;39:277–81. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

