Comparison of the inhibitory activities of human tissue factor pathway inhibitor (TFPI)α and TFPIβ
- PMID: 23480518
- PMCID: PMC3656975
- DOI: 10.1111/jth.12188
Comparison of the inhibitory activities of human tissue factor pathway inhibitor (TFPI)α and TFPIβ
Abstract
Background: Tissue factor pathway inhibitor (TFPI) is an alternatively spliced protein with two isoforms, TFPIα and TFPIβ, which differ in their C-terminal structure and cellular localization. Detailed characterization of their inhibitory activity is needed to define potentially unique inhibitory roles in tissue factor (TF)-mediated thrombotic and inflammatory disease, and to understand how pharmaceuticals targeted to different structural regions of the TFPI isoforms alter hemostasis in hemophilia patients.
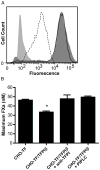
Methods: The TF inhibitory activity of TFPIβ localized to the surface of CHO cells was compared with that of soluble TFPIα by the use of in vitro and in vivo assays.
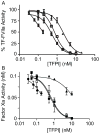
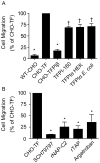
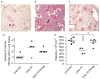
Results: In TF-factor VIIa-mediated FXa generation assays, TFPIβ was a slightly better inhibitor than TFPIα, which was approximately three-fold better than TFPI-160, a soluble, altered form of TFPI similar to TFPIβ. In direct FXa inhibitory assays, TFPIβ had an IC50 2.5-fold lower than that of TFPIα and 56-fold lower than that of TFPI-160. TFPIβ inhibited TF-mediated CHO cell migration though Matrigel, whereas TFPIα and TFPI-160 were poor inhibitors, demonstrating that TFPIβ effectively blocks TF-initiated signaling events during cellular migration through matrices that are not permeable to soluble forms of TFPI. Furthermore, TFPIβ inhibited TF-dependent CHO cell infiltration into lung tissue following tail vein injection into SCID mice, and blocked the development of consumptive coagulopathy.
Conclusions: TFPIβ is a slightly better inhibitor of TF procoagulant activity than TFPIα. As a surface-associated protein, TFPIβ is a much better inhibitor of TF-mediated cellular migration than soluble TFPIα, and may specifically act in the inhibition of TF-mediated signaling events on inflamed endothelium and/or monocytes.
© 2013 International Society on Thrombosis and Haemostasis.
Conflict of interest statement
This work was supported by NHLBI grants HL068835 to AEM and HL091469 to SAM. JPW gratefully acknowledges training grant support from NHLBI grant HL007209. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Further support was provided through a research grant from Novo Nordisk to AEM.
Figures
References
-
- Ratnoff OD, Davie EW. The Activation of Christmas Factor (Factor IX) by Activated Plasma Thromboplastin Antecedent (Activated Factor XI)*. Biochemistry. 1962;1:677–85.
-
- Girard TJ, Warren LA, Novotny WF, Likert KM, Brown SG, Miletich JP, Broze GJ., Jr Functional significance of the Kunitz-type inhibitory domains of lipoprotein-associated coagulation inhibitor. Nature. 1989;338:518–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

