The clinical benefit of ruxolitinib across patient subgroups: analysis of a placebo-controlled, Phase III study in patients with myelofibrosis
- PMID: 23480528
- PMCID: PMC4055021
- DOI: 10.1111/bjh.12274
The clinical benefit of ruxolitinib across patient subgroups: analysis of a placebo-controlled, Phase III study in patients with myelofibrosis
Abstract
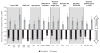
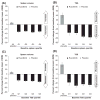
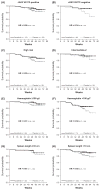
Myelofibrosis (MF) patients can present with a wide spectrum of disease characteristics. We analysed the consistency of ruxolitinib efficacy across patient subgroups in the COntrolled MyeloFibrosis Study With ORal JAK Inhibitor Treatment (COMFORT-I,) a double-blind trial, where patients with intermediate-2 or high-risk MF were randomized to twice-daily oral ruxolitinib (n = 155) or placebo (n = 154). Subgroups analysed included MF subtype (primary, post-polycythaemia vera, post-essential thrombocythaemia), age (≤65, > 65 years), International Prognostic Scoring System risk group, baseline Eastern Cooperative Oncology Group performance status (0, 1, ≥2), JAK2 V617F mutation (positive, negative), baseline haemoglobin level (≥100, <100 g/l), baseline platelet count (100-200 × 10(9)/l, >200 × 10(9)/l), baseline palpable spleen size (≤10, >10 cm), and baseline quartile of spleen volume and Total Symptom Score (TSS; Q1 = lowest, Q4 = highest). Mean percentage change from baseline to week 24 in spleen volume and TSS were calculated for ruxolitinib and placebo in each subgroup. Overall survival was estimated by Kaplan-Meier method according to original randomization group. In ruxolitinib-treated patients, reductions in spleen volume and TSS and evidence of improved survival relative to placebo across subgroups were consistent with those seen in the COMFORT-I population, confirming that ruxolitinib is an effective therapy for the spectrum of MF patients studied in COMFORT-I.
© 2013 John Wiley & Sons Ltd.
Conflict of interest statement
S.V. reports receiving grant support through his institution from Incyte Corporation, Exelixis, Celgene, NS Pharma, Infinity Pharmaceuticals, SBIO, Lilly Oncology, AstraZeneca, Geron, Bristol-Myers Squibb, YM BioSciences, Gilead and Roche; RAM., receiving research funding from Incyte Corporation, Lilly, Sanofi, NS Pharma and YM Bioscience; JG., receiving Consultancy, Honoraria and Support for travel to meeting for the study or other purposes from Incyte Corporation; R.S.L., is an employee of Incyte Corporation and owns stock in Incyte Corporation; V.G., receiving grant support through his institution from Incyte Corporation and Novartis, consulting fees from Incyte Corporation and Novartis, and lecture fees from Novartis; J.F.P. has no relationships to disclose; J.V.C., receiving consulting fees from Incyte Corporation; M.D., receiving consulting fees from Bristol-Myers Squibb, Novartis and Ariad, and grant support through his institution from Bristol-Myers Squibb; C.M., receiving grant support through her institution, consulting fees, and lecture fees from Novartis, and payments for development of educational presentations from Incyte Corporation and Novartis; R.T.S., receiving grant support through his institution from Incyte Corporation and lecture fees from Incyte Corporation, and holding stock in Incyte Corporation both individually and through his institution; M.T., receiving membership on an entity’s Board of Directors or advisory committees from Novartis, BMS, Sanofi, Teva and Pfizer and research funding from Novartis, BMS, Ariad, and Sanofi and speakers bureau for Novartis; E.F.W., receiving consultancy and honoraria from Incyte Corporation; J.H.H. has no relationships to disclose; M.O.A., receiving research funding from Incyte Corporation; E.H., has no relationships to disclose; R.M.L., receiving grant support through his institution from Incyte Corporation; R.P., receiving consulting fees paid through his institution from Incyte Corporation; A.R., has no relationships to disclose; K.V., W.S. and V.S. are employees of Incyte Corporation and own stock in Incyte Corporation; S.E.-V. is a former employee of Incyte Corporation and owns stock in Incyte Corporation. R.M.K., receiving grant support through his institution from Incyte Corporation.
Figures


References
-
- Barbui T, Barosi G, Birgegard G, Cervantes F, Finazzi G, Griesshammer M, Harrison C, Hasselbalch HC, Hehlmann R, Hoffman R, Kiladjian JJ, Kroger N, Mesa R, McMullin MF, Pardanani A, Passamonti F, Vannucchi AM, Reiter A, Silver RT, Verstovsek S, Tefferi A European Leukemia Net. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. Journal of Clinical Oncology. 2011;29:761–770. - PMC - PubMed
-
- Barosi G, Mesa RA, Thiele J, Cervantes F, Campbell PJ, Verstovsek S, Dupriez B, Levine RL, Passamonti F, Gotlib J, Reilly JT, Vannucchi AM, Hanson CA, Solberg LA, Orazi A, Tefferi A International Working Group for Myelofibrosis Research Treatment (IWG-MR) Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a consensus statement from the International Working Group for Myelofibrosis Research and Treatment. Leukemia. 2008;22:437–438. - PubMed
-
- Cervantes F, Pereira A, Esteve J, Rafel M, Cobo F, Rozman C, Montserrat E. Identification of ‘short-lived’ and ‘long-lived’ patients at presentation of idiopathic myelofibrosis. British Journal of Haematology. 1997;97:635–640. - PubMed
-
- Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E, Vannucchi AM, Mesa RA, Demory JL, Barosi G, Rumi E, Tefferi A. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113:2895–2901. - PubMed
-
- Cuzick J. Forest plots and the interpretation of subgroups. Lancet. 2005;365:1308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

