p57 controls adult neural stem cell quiescence and modulates the pace of lifelong neurogenesis
- PMID: 23481253
- PMCID: PMC3616292
- DOI: 10.1038/emboj.2013.50
p57 controls adult neural stem cell quiescence and modulates the pace of lifelong neurogenesis
Abstract
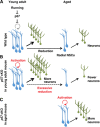
Throughout life, neural stem cells (NSCs) in the adult hippocampus persistently generate new neurons that modify the neural circuitry. Adult NSCs constitute a relatively quiescent cell population but can be activated by extrinsic neurogenic stimuli. However, the molecular mechanism that controls such reversible quiescence and its physiological significance have remained unknown. Here, we show that the cyclin-dependent kinase inhibitor p57(kip2) (p57) is required for NSC quiescence. In addition, our results suggest that reduction of p57 protein in NSCs contributes to the abrogation of NSC quiescence triggered by extrinsic neurogenic stimuli such as running. Moreover, deletion of p57 in NSCs initially resulted in increased neurogenesis in young adult and aged mice. Long-term p57 deletion, on the contrary, led to NSC exhaustion and impaired neurogenesis in aged mice. The regulation of NSC quiescence by p57 might thus have important implications for the short-term (extrinsic stimuli-dependent) and long-term (age-related) modulation of neurogenesis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






References
-
- Ahn S, Joyner AL (2005) In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature 437: 894–897 - PubMed
-
- Ben Abdallah NM, Slomianka L, Vyssotski AL, Lipp HP (2010) Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol Aging 31: 151–161 - PubMed
-
- Bondolfi L, Ermini F, Long JM, Ingram DK, Jucker M (2004) Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiol Aging 25: 333–340 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

