Intradermal adipocytes mediate fibroblast recruitment during skin wound healing
- PMID: 23482487
- PMCID: PMC3596993
- DOI: 10.1242/dev.087593
Intradermal adipocytes mediate fibroblast recruitment during skin wound healing
Abstract
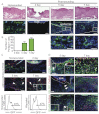
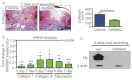
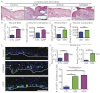
Acute wound healing in the skin involves the communication of multiple cell types to coordinate keratinocyte and fibroblast proliferation and migration for epidermal and dermal repair. Many studies have focused on the interplay between hematopoietic cells, keratinocytes and fibroblasts during skin wound healing, yet the possible roles for other cell types within the skin, such as intradermal adipocytes, have not been investigated during this process. Here, we identify that adipocyte lineage cells are activated and function during acute skin wound healing. We find that adipocyte precursor cells proliferate and mature adipocytes repopulate skin wounds following inflammation and in parallel with fibroblast migration. Functional analysis of mice with defects in adipogenesis demonstrates that adipocytes are necessary for fibroblast recruitment and dermal reconstruction. These data implicate adipocytes as a key component of the intercellular communication that mediates fibroblast function during skin wound healing.
Figures




References
-
- Arnold M., Barbul A. (2006). Nutrition and wound healing. Plast. Reconstr. Surg. 117 Suppl., 42S–58S - PubMed
-
- Blanpain C., Lowry W. E., Geoghegan A., Polak L., Fuchs E. (2004). Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118, 635–648 - PubMed
-
- Brack A. S., Conboy M. J., Roy S., Lee M., Kuo C. J., Keller C., Rando T. A. (2007). Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317, 807–810 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

