Autologous lysate-pulsed dendritic cell vaccination followed by adoptive transfer of vaccine-primed ex vivo co-stimulated T cells in recurrent ovarian cancer
- PMID: 23482679
- PMCID: PMC3583933
- DOI: 10.4161/onci.22664
Autologous lysate-pulsed dendritic cell vaccination followed by adoptive transfer of vaccine-primed ex vivo co-stimulated T cells in recurrent ovarian cancer
Abstract
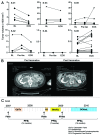
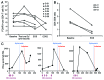
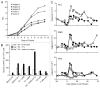
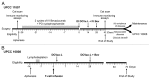
Novel strategies for the therapy of recurrent ovarian cancer are warranted. We report a study of a combinatorial approach encompassing dendritic cell (DC)-based autologous whole tumor vaccination and anti-angiogenesis therapy, followed by the adoptive transfer of autologous vaccine-primed CD3/CD28-co-stimulated lymphocytes. Recurrent ovarian cancer patients for whom tumor lysate was available from prior cytoreductive surgery underwent conditioning with intravenous bevacizumab and oral metronomic cyclophosphamide, sequentially followed by (1) bevacizumab plus vaccination with DCs pulsed with autologous tumor cell lysate supernatants, (2) lymphodepletion and (3) transfer of 5 × 109 autologous vaccine-primed T-cells in combination with the vaccine. Feasibility, safety as well as immunological and clinical efficacy were evaluated. Six subjects received this vaccination. Therapy was feasible, well tolerated, and elicited antitumor immune responses in four subjects, who also experienced clinical benefits. Of these, three patients with residual measurable disease received outpatient lymphodepletion and adoptive T-cell transfer, which was well tolerated and resulted in a durable reduction of circulating regulatory T cells and increased CD8+ lymphocyte counts. The vaccine-induced restoration of antitumor immunity was achieved in two subjects, who also demonstrated clinical benefits, including one complete response. Our findings indicate that combinatorial cellular immunotherapy for the treatment of recurrent ovarian cancer is well tolerated and warrants further investigation. Several modifications of this approach can be envisioned to optimize immunological and clinical outcomes.
Keywords: DC vaccines; adoptive T-cell transfer; autologous immunotherapy.
Figures

References
-
- Schlienger K, Chu CS, Woo EY, Rivers PM, Toll AJ, Hudson B, et al. TRANCE- and CD40 ligand-matured dendritic cells reveal MHC class I-restricted T cells specific for autologous tumor in late-stage ovarian cancer patients. Clin Cancer Res. 2003;9:1517–27. - PubMed
-
- Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–43. doi: 10.1073/pnas.0509182102. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
