Protein import into the photosynthetic organelles of Paulinella chromatophora and its implications for primary plastid endosymbiosis
- PMID: 23482692
- PMCID: PMC3589627
- DOI: 10.1007/s13199-012-0202-2
Protein import into the photosynthetic organelles of Paulinella chromatophora and its implications for primary plastid endosymbiosis
Abstract
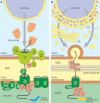
The rhizarian amoeba Paulinella chromatophora harbors two photosynthetically active organelles of cyanobacterial origin that have been acquired independently of classic primary plastids. Because their acquisition did take place relatively recently, they are expected to provide new insight into the ancient cyanobacterial primary endosymbiosis. During the process of Paulinella endosymbiont-to-organelle transformation, more than 30 genes have been transferred from the organelle to the host nuclear genome via endosymbiotic gene transfer (EGT). The article discusses step-by-step protein import of EGT-derived proteins into Paulinella photosynthetic organelles with the emphasis on the nature of their targeting signals and the final passage of proteins through the inner organelle membrane. The latter most probably involves a simplified Tic translocon composed of Tic21- and Tic32-like proteins as well as a Hsp70-based motor responsible for pulling of imported proteins into the organelle matrix. Our results indicate that although protein translocation across the inner membrane of Paulinella photosynthetic organelles seems to resemble the one in classic primary plastids, the transport through the outer membrane does not. The differences could result from distinct integration pathways of Paulinella photosynthetic organelles and primary plastids with their respective host cells.
Keywords: Endosymbiosis; Molecular motor; Plastid; Protein import; Signal peptide; Vesicular trafficking.
Figures


References
-
- Adl SM, Simpson AG, Lane CE, Lukes J, Bass D, Bowser SS, Brown MW, Burki F, Dunthorn M, Hampl V, Heiss A, Hoppenrath M, Lara E, le Gall L, Lynn DH, McManus H, Mitchell EA, Mozley-Stanridge SE, Parfrey LW, Pawlowski J, Rueckert S, Shadwick L, Schoch CL, Smirnov A, Spiegel FW. The revised classification of eukaryotes. J Eukaryot Microbiol. 2012;59:429–514. doi: 10.1111/j.1550-7408.2012.00644.x. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
