Protein S-ACYL Transferase10 is critical for development and salt tolerance in Arabidopsis
- PMID: 23482856
- PMCID: PMC3634679
- DOI: 10.1105/tpc.112.108829
Protein S-ACYL Transferase10 is critical for development and salt tolerance in Arabidopsis
Abstract
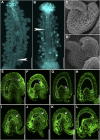
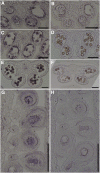
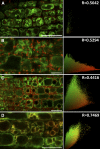
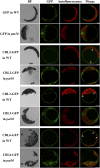
Protein S-acylation, commonly known as palmitoylation, is a reversible posttranslational modification that catalyzes the addition of a saturated lipid group, often palmitate, to the sulfhydryl group of a Cys. Palmitoylation regulates enzyme activity, protein stability, subcellular localization, and intracellular sorting. Many plant proteins are palmitoylated. However, little is known about protein S-acyl transferases (PATs), which catalyze palmitoylation. Here, we report that the tonoplast-localized PAT10 is critical for development and salt tolerance in Arabidopsis thaliana. PAT10 loss of function resulted in pleiotropic growth defects, including smaller leaves, dwarfism, and sterility. In addition, pat10 mutants are hypersensitive to salt stresses. We further show that PAT10 regulates the tonoplast localization of several calcineurin B-like proteins (CBLs), including CBL2, CBL3, and CBL6, whose membrane association also depends on palmitoylation. Introducing a C192S mutation within the highly conserved catalytic motif of PAT10 failed to complement pat10 mutants, indicating that PAT10 functions through protein palmitoylation. We propose that PAT10-mediated palmitoylation is critical for vacuolar function by regulating membrane association or the activities of tonoplast proteins.
Figures





References
-
- Apse M.P., Sottosanto J.B., Blumwald E. (2003). Vacuolar cation/H+ exchange, ion homeostasis, and leaf development are altered in a T-DNA insertional mutant of AtNHX1, the Arabidopsis vacuolar Na+/H+ antiporter. Plant J. 36: 229–239 - PubMed
-
- Baekkeskov S., Kanaani J. (2009). Palmitoylation cycles and regulation of protein function (Review). Mol. Membr. Biol. 26: 42–54 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

