Betting on improved cancer immunotherapy by doubling down on CD134 and CD137 co-stimulation
- PMID: 23482891
- PMCID: PMC3583935
- DOI: 10.4161/onci.22837
Betting on improved cancer immunotherapy by doubling down on CD134 and CD137 co-stimulation
Abstract
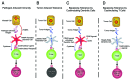
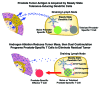
The ability of T cells to recognize a vast array of antigens enables them to destroy tumor cells while inflicting minimal collateral damage. Nevertheless, tumor antigens often are a form of self-antigen, and thus tumor immunity can be dampened by tolerance mechanisms that evolved to prevent autoimmunity. Since tolerance can be induced by steady-state antigen-presenting cells that provide insufficient co-stimulation, the exogenous administration of co-stimulatory agonists can favor the expansion and tumoricidal functions of tumor-specific T cells. Agonists of the co-stimulatory tumor necrosis factor receptor (TNFR) family members CD134 and CD137 exert antitumor activity in mice, and as monotherapies have exhibited encouraging results in clinical trials. This review focuses on how the dual administration of CD134 and CD137 agonists synergistically boosts T-cell priming and elaborates a multi-pronged antitumor immune response, as well as how such dual co-stimulation might be translated into effective anticancer therapies.
Keywords: CD134; CD137; CD4 T cell; dual costimulation; immunotherapy.
Figures
Similar articles
-
Tumor-Unrelated CD4 T Cell Help Augments CD134 plus CD137 Dual Costimulation Tumor Therapy.J Immunol. 2015 Dec 15;195(12):5816-26. doi: 10.4049/jimmunol.1502032. Epub 2015 Nov 11. J Immunol. 2015. PMID: 26561553 Free PMC article.
-
CD134/CD137 dual costimulation-elicited IFN-γ maximizes effector T-cell function but limits Treg expansion.Immunol Cell Biol. 2013 Feb;91(2):173-83. doi: 10.1038/icb.2012.74. Epub 2013 Jan 8. Immunol Cell Biol. 2013. PMID: 23295363 Free PMC article.
-
Expression and costimulatory effects of the TNF receptor superfamily members CD134 (OX40) and CD137 (4-1BB), and their role in the generation of anti-tumor immune responses.Eur J Immunol. 2002 Dec;32(12):3617-27. doi: 10.1002/1521-4141(200212)32:12<3617::AID-IMMU3617>3.0.CO;2-M. Eur J Immunol. 2002. PMID: 12516549
-
Harnessing co-stimulatory TNF receptors for cancer immunotherapy: Current approaches and future opportunities.Hum Antibodies. 2017;25(3-4):87-109. doi: 10.3233/HAB-160308. Hum Antibodies. 2017. PMID: 28085016 Review.
-
Agonists of Co-stimulation in Cancer Immunotherapy Directed Against CD137, OX40, GITR, CD27, CD28, and ICOS.Semin Oncol. 2015 Aug;42(4):640-55. doi: 10.1053/j.seminoncol.2015.05.014. Epub 2015 Jun 11. Semin Oncol. 2015. PMID: 26320067 Review.
Cited by
-
T-cell co-stimulation in combination with targeting FAK drives enhanced anti-tumor immunity.Elife. 2020 Jan 21;9:e48092. doi: 10.7554/eLife.48092. Elife. 2020. PMID: 31959281 Free PMC article.
-
Combination cancer immunotherapy and new immunomodulatory targets.Nat Rev Drug Discov. 2015 Aug;14(8):561-84. doi: 10.1038/nrd4591. Nat Rev Drug Discov. 2015. PMID: 26228759 Review.
-
Recent Advances in Cancer Vaccines: Challenges, Achievements, and Futuristic Prospects.Vaccines (Basel). 2022 Nov 25;10(12):2011. doi: 10.3390/vaccines10122011. Vaccines (Basel). 2022. PMID: 36560420 Free PMC article. Review.
-
Trial Watch: Immunomodulatory monoclonal antibodies for oncological indications.Oncoimmunology. 2015 Mar 2;4(4):e1008814. doi: 10.1080/2162402X.2015.1008814. eCollection 2015 Apr. Oncoimmunology. 2015. PMID: 26137403 Free PMC article. Review.
-
New Combination Strategies Using Programmed Cell Death 1/Programmed Cell Death Ligand 1 Checkpoint Inhibitors as a Backbone.Cancer J. 2017 Jan/Feb;23(1):10-22. doi: 10.1097/PPO.0000000000000246. Cancer J. 2017. PMID: 28114250 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
