Antibiotic-Releasing Silk Biomaterials for Infection Prevention and Treatment
- PMID: 23483738
- PMCID: PMC3589549
- DOI: 10.1002/adfm.201201636
Antibiotic-Releasing Silk Biomaterials for Infection Prevention and Treatment
Abstract
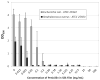
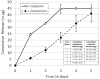
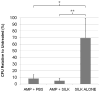
Effective treatment of infections in avascular and necrotic tissues can be challenging due to limited penetration into the target tissue and systemic toxicities. Controlled release polymer implants have the potential to achieve the high local concentrations needed while also minimizing systemic exposure. Silk biomaterials possess unique characteristics for antibiotic delivery including biocompatibility, tunable biodegradation, stabilizing effects, water-based processing and diverse material formats. We report on functional release of antibiotics spanning a range of chemical properties from different material formats of silk (films, microspheres, hydrogels, coatings). The release of penicillin and ampicillin from bulk-loaded silk films, drug-loaded silk microspheres suspended in silk hydrogels and bulk-loaded silk hydrogels was investigated and in vivo efficacy of ampicillin-releasing silk hydrogels was demonstrated in a murine infected wound model. Silk sponges with nanofilm coatings were loaded with gentamicin and cefazolin and release was sustained for 5 and 3 days, respectively. The capability of silk antibiotic carriers to sequester, stabilize and then release bioactive antibiotics represents a major advantage over implants and pumps based on liquid drug reservoirs where instability at room or body temperature is limiting. The present studies demonstrate that silk biomaterials represent a novel, customizable antibiotic platform for focal delivery of antibiotics using a range of material formats (injectable to implantable).
Keywords: antibiotics; biological applications of polymers; biomaterials; drug delivery systems; silk fibroin.
Conflict of interest statement
The authors have no competing financial interests to declare.
Figures




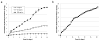
References
-
- Nandi SK, Mukherjee P, Roy S, Kundu B, De DK, Basu D. Mater Sci Eng C. 2009;29:2478.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
