Mechanisms of cyclic nucleotide phosphodiesterases in modulating T cell responses in murine graft-versus-host disease
- PMID: 23483980
- PMCID: PMC3590136
- DOI: 10.1371/journal.pone.0058110
Mechanisms of cyclic nucleotide phosphodiesterases in modulating T cell responses in murine graft-versus-host disease
Abstract
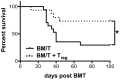
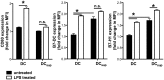
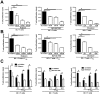
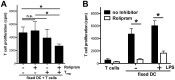
Graft-versus-host disease (GvHD) is a key contributor to the morbidity and mortality after allogeneic hematopoetic stem cell transplantation (HSCT). Regulatory Foxp3(+) CD4(+) T cells (Treg) suppress conventional T cell activation and can control GvHD. In our previous work, we demonstrate that a basic mechanism of Treg mediated suppression occurs by the transfer of cyclic adenosine monophosphate (cAMP) to responder cells. Whether this mechanism is relevant for Treg mediated suppression of GvHD is currently unknown. To address this question, bone marrow and T cells from C57BL/6 mice were transferred into lethally irradiated BALB/c recipients, and the course of GvHD and survival were monitored. Transplanted recipients developed severe GvHD that was strongly ameliorated by the transfer of donor Treg cells. Towards the underlying mechanisms, in vitro studies revealed that Treg communicated with DCs via gap junctions, resulting in functional inactivation of DC by a metabolic pathway involving cAMP that is modulated by the phosphodiesterase (PDE) 4 inhibitor rolipram. PDE2 or PDE3 inhibitors as well as rolipram suppressed allogeneic T cell activation, indirectly by enhancing Treg mediated suppression of DC activation and directly by inhibiting responder T cell proliferation. In line with this, we observed a cooperative suppression of GvHD upon Treg transfer and additional rolipram treatment. In conclusion, we propose that an important pathway of Treg mediated control of GvHD is based on a cAMP dependent mechanism. These data provide the basis for future concepts to manipulate allogeneic T cell responses to prevent GvHD.
Conflict of interest statement
Figures


References
-
- Doehner H, Estey EH, Amadori S, Appelbaum FR, Buechner T, et al. (2010) Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 115: 453–474 doi:10.1182/blood-2009-07-235358. - DOI - PubMed
-
- KOLB H, SCHATTENBERG A, GOLDMAN J, HERTENSTEIN B, JACOBSEN N, et al. (1995) Graft-Versus-Leukemia Effect of Donor Lymphocyte Transfusions in Marrow Grafted Patients. Blood 86: 2041–2050. - PubMed
-
- Ferrara JLM, Levine JE, Reddy P, Holler E (2009) Graft-versus-host disease. Lancet 373: 1550–1561 doi:10.1016/S0140-6736(09)60237-3. - DOI - PMC - PubMed
-
- Sakaguchi S (2004) Naturally Arising CD4+ Regulatory T Cells for Immunologic Self-Tolerance and Negative Control of Immune Responses. Ann Rev Immunol 22: 531–562. - PubMed
-
- Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, et al. (1999) Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res 59: 3128–3133. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

