The steady-state serum concentration of genistein aglycone is affected by formulation: a bioequivalence study of bone products
- PMID: 23484100
- PMCID: PMC3591111
- DOI: 10.1155/2013/273498
The steady-state serum concentration of genistein aglycone is affected by formulation: a bioequivalence study of bone products
Abstract
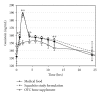
An FDA-regulated, prescription medical food (Fosteum; 27 mg natural genistein, 200 IU cholecalciferol, 20 mg citrated zinc bisglycinate (4 mg elemental zinc) per capsule) and an over-the-counter (OTC) supplement (Citracal Plus Bone Density Builder; 27 mg synthetic genistein, 600 mg elemental calcium (calcium citrate), 400 IU vitamin D3, 50 mg magnesium, 7.5 mg zinc, 1 mg copper, 75 μ g molybdenum, 250 μ g boron per two tablets) were compared to a clinically proven bone formulation (27 mg natural genistein, 400 IU cholecalciferol, 500 mg elemental calcium (calcium carbonate) per tablet; the Squadrito formulation) in an 8-day steady-state pharmacokinetic (PK) study of healthy postmenopausal women (n = 30) randomized to receive 54 mg of genistein per day. Trough serum samples were obtained before the final dose on the morning of the ninth day followed by sampling at 1, 2, 4, 6, 8, 10, 12, 24, 36, 48, 72, and 96 hrs. Total serum genistein, after β -glucuronidase/sulfatase digestion, was measured by time-resolved fluorometric assay. Maximal time (Tmax), concentration (Cmax), half-life (T1/2), and area under the curve (AUC) were determined for genistein in each formulation. Fosteum and the Squadrito study formulation were equivalent for genistein Tmax (2 hrs), Cmax (0.7 μM), T1/2 (18 ± 6.9 versus 21 ± 4.9 hrs), and AUC (9221 ± 413 versus 9818 ± 1370 ng·hr/mL). The OTC supplement's synthetically derived genistein, however, showed altered Tmax (6 hrs), Cmax (0.57 μ M), T1/2 (8.3 ± 1.9 hrs), and AUC (6474 ± 287 ng·hr/mL). Differences in uptake may be due to multiple ingredients in the OTC supplement which interfere with genistein absorption.
Figures


References
-
- Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutrition and Cancer. 2006;55(1):1–12. - PubMed
-
- De Kleijn MJJ, Van Der Schouw YT, Wilson PWF, et al. Intake of dietary phytoestrogens is low in postmenopausal women in the United States: the framingham study1-4 . Journal of Nutrition. 2001;131(6):1826–1832. - PubMed
-
- Horn-Ross PL, John EM, Lee M, et al. Phytoestrogen consumption and breast cancer risk in a multiethnic population: the bay area breast cancer study. American Journal of Epidemiology. 2001;154(5):434–441. - PubMed
-
- Zhang X, Shu XO, Li H, et al. Prospective cohort study of soy food consumption and risk of bone fracture among postmenopausal women. Archives of Internal Medicine. 2005;165(16):1890–1895. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

