Islet inflammation: a unifying target for diabetes treatment?
- PMID: 23484621
- PMCID: PMC3686848
- DOI: 10.1016/j.tem.2013.01.007
Islet inflammation: a unifying target for diabetes treatment?
Abstract
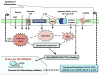
In the past decade, islet inflammation has emerged as a contributor to the loss of functional β cell mass in both type 1 (T1D) and type 2 diabetes (T2D). Evidence supports the idea that overnutrition and insulin resistance result in the production of proinflammatory mediators by β cells. In addition to compromising β cell function and survival, cytokines may recruit macrophages into islets, thus augmenting inflammation. Limited but intriguing data imply a role of adaptive immune response in islet dysfunction in T2D. Clinical trials have validated anti-inflammatory therapies in T2D, whereas immune therapy for T1D remains challenging. Further research is required to improve our understanding of islet inflammatory pathways and to identify more effective therapeutic targets for T1D and T2D.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

References
-
- Das A, Mukhopadhyay S. The evil axis of obesity, inflammation and type-2 diabetes. Endocrine, metabolic & immune disorders drug targets. 2011;11:23–31. - PubMed
-
- Verbeeten KC, et al. Association between childhood obesity and subsequent Type 1 diabetes: a systematic review and meta-analysis. Diabetic medicine: a journal of the British Diabetic Association. 2011;28:10–18. - PubMed
-
- Larsen CM, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. The New England journal of medicine. 2007;356:1517–1526. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

