Retained surgical sponges, needles and instruments
- PMID: 23484986
- PMCID: PMC4098594
- DOI: 10.1308/003588413X13511609957218
Retained surgical sponges, needles and instruments
Abstract
Introduction: Retained sponges and instruments (RSI) due to surgery are a recognised medical 'never event' and have catastrophic implications for patients, healthcare professionals and medical care providers. The aim of this review was to elucidate the extent of the problem of RSI and to identify preventative strategies.
Methods: A comprehensive literature search was performed on MEDLINE(®), Embase™, the Science Citation Index and Google™ Scholar for articles published in English between January 2000 and June 2012. Studies outlining the incidence, risk, management and attempts to prevent RSI following surgical intervention were retrieved.
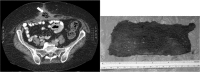
Results: The overall incidence of RSI is low although its incidence is substantially higher in operations performed on open cavities. Sponges are the most commonly retained item when compared with needles and instruments. Clinical presentation is varied, leading to avoidable morbidity, and the error is indefensible medicolegally. Risk factors include emergency operations, operations involving unexpected change in procedure, raised body mass index, and a failure to perform accurate sponge and instrument counts. The existing strategy for prevention is manual counting of sponges and instruments undertaken by surgical personnel. This, however, is fallible. Computer assisted counting of sponges using barcodes and gauze sponges tagged with a radiofrequency identification device aiding manual counting have been trialled recently, with success.
Conclusions: Vigilance among operating theatre personnel is paramount if RSI is to be prevented. Prospective multicentre trials to assess efficacy of new technologies aiding manual counting should be undertaken if this medical error is to be eliminated completely.
Figures
Comment in
-
Retained surgical sponges, needles and instruments.Ann R Coll Surg Engl. 2014 Mar;96(2):174-5. doi: 10.1308/rcsann.2014.174. Ann R Coll Surg Engl. 2014. PMID: 24780693 Free PMC article. No abstract available.
-
Authors' response.Ann R Coll Surg Engl. 2014 Mar;96(2):175. doi: 10.1308/rcsann.2014.175. Ann R Coll Surg Engl. 2014. PMID: 24895764 Free PMC article. No abstract available.
References
-
- National Quality Forum. Serious Reportable Events In Healthcare – 2011 Update. Washington DC: NQF: 2011
-
- Department of Health. The ‘Never Events’ List 2011/12. London: DH; 2011
-
- Gawande AA, Studdert DM, Orav EJet al Risk factors for retained instruments and sponges after surgery. N Engl J Med 2003; 348: 229–235 - PubMed
-
- Berkowitz S, Marshall H, Charles A. Retained intra-abdominal surgical instruments: time to use nascent technology? Am Surg 2007; 73: 1,083–1,085 - PubMed
-
- Dippolito A, Braslow BM, Lombardo Get al How David beat Goliath: history of physicians fighting frivolous lawsuits. OPUS 12 Scientist 2008; 2: 1–8
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

