Cost utility of lateral-flow urine lipoarabinomannan for tuberculosis diagnosis in HIV-infected African adults
- PMID: 23485389
- PMCID: PMC3918209
- DOI: 10.5588/ijtld.12.0627
Cost utility of lateral-flow urine lipoarabinomannan for tuberculosis diagnosis in HIV-infected African adults
Abstract
Setting: In-patient hospitals in South Africa and Uganda.
Objective: To evaluate the cost-effectiveness of a lateral-flow urine lipoarabinomannan (LAM) test when added to existing strategies for tuberculosis (TB) diagnosis in human immunodeficiency virus infected adults (CD4(+) T-cell counts < 100 cells/l) with symptoms of active TB.
Design: Decision-analytic cost-utility model, with the primary outcome being the incremental cost-effectiveness ratio, expressed in 2010 US dollars per disability-adjusted life year (DALY) averted from the perspective of a public sector TB control program.
Results and conclusion: For every 1000 patients tested, adding lateral-flow urine LAM generated 80 incremental appropriate anti-tuberculosis treatments and averted 224 DALYs. Estimated cost utility was US$353 per DALY averted (95% uncertainty range $192$1161) in South Africa and $86 per DALY averted (95% uncertainty range $49$239) in Uganda, reflecting the lower treatment costs in Uganda. Cost utility was most sensitive to assay specificity, cost of anti-tuberculosis treatment, life expectancy after TB cure and cohort TB prevalence, but did not rise above $1500 per DALY averted in South Africa under any one-way sensitivity analysis. The probability of acceptability was >99.8% at a per-DALY willingness-to-pay threshold equal to the per capita gross domestic product in South Africa ($7275) and Uganda ($509).
Figures

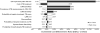

References
-
- Bock NN, Jensen PA, Miller B, Nardell E. Tuberculosis infection control in resource-limited settings in the era of expanding HIV care and treatment. J. Infect. Dis. 2007;196(Suppl 1):S108–113. - PubMed
-
- Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection-associated tuberculosis: the epidemiology and the response. Clin. Infect. Dis. 2010;50(Suppl 3):S201–207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

