Has the tobacco industry evaded the FDA's ban on 'Light' cigarette descriptors?
- PMID: 23485704
- PMCID: PMC3932763
- DOI: 10.1136/tobaccocontrol-2012-050746
Has the tobacco industry evaded the FDA's ban on 'Light' cigarette descriptors?
Abstract
Background: Under the Family Smoking Prevention and Tobacco Control Act (FSPTCA), the Food and Drug Administration (FDA) banned the use of "Lights" descriptors or similar terms on tobacco products that convey messages of reduced risk. Manufacturers eliminated terms explicitly stated and substituted colour name descriptors corresponding to the banned terms. This paper examines whether the tobacco industry complied with or circumvented the law and potential FDA regulatory actions.
Methods: Philip Morris retailer manuals, manufacturers' annual reports filed with the Massachusetts Department of Public Health, a national public opinion survey, and market-wide cigarette sales data were examined.
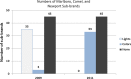
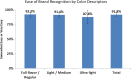
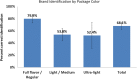
Results: Manufacturers substituted "Gold" for "Light" and "Silver" for "Ultra-light" in the names of Marlboro sub-brands, and "Blue", "Gold", and "Silver" for banned descriptors in sub-brand names. Percent filter ventilation levels, used to generate the smoke yield ranges associated with "Lights" categories, appear to have been reassigned to the new colour brand name descriptors. Following the ban, 92% of smokers reported they could easily identify their usual brands, and 68% correctly named the package colour associated with their usual brand, while sales for "Lights" cigarettes remained unchanged.
Conclusions: Tobacco manufacturers appear to have evaded a critical element of the FSPTCA, the ban on misleading descriptors that convey reduced health risk messages. The FPSTCA provides regulatory mechanisms, including banning these products as adulterated (Section 902). Manufacturers could then apply for pre-market approval as new products and produce evidence for FDA evaluation and determination whether or not sales of these products are in the public health interest.
Keywords: Packaging and Labelling; Public Opinion; Public Policy; Tobacco Industry.
Figures
References
-
- FDA Science and Mission at Risk: Report of the Subcommittee on Science and Technology. FDA Science Board, Nov 2007. http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-329b_02_01_FDA%20Re... (accessed 24 aug 2012).
-
- Family Smoking Prevention and Tobacco Control Act, Pub L. 111–31, 123 Stat 1776 (2009)
-
- Carpenter D. Reputation and power: organizational image and pharmaceutical regulation at the FDA. New Jersey: Princeton University Press, 2010
-
- Final Opinion, United States v. Philip Morris USA, Inc Civil Action No. 99–2496, 17 August 2006, page 1631. http://www.library.ucsf.edu/sites/all/files/ucsf_assets/FinalOpinion_ful... (accessed 17 Jul 2012).
-
- U.S. Public Health Service. Smoking and health: A report of the advisory committee to the Surgeon General of the Public Health Service. U.S. Department of Health, Education, and Welfare. Public Health Service. Centers for Disease Control. PHS Publication No. 1103. 1964.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical