Multiple phases of chondrocyte enlargement underlie differences in skeletal proportions
- PMID: 23485973
- PMCID: PMC3606657
- DOI: 10.1038/nature11940
Multiple phases of chondrocyte enlargement underlie differences in skeletal proportions
Abstract
The wide diversity of skeletal proportions in mammals is evident upon a survey of any natural history museum's collections and allows us to distinguish between species even when reduced to their calcified components. Similarly, each individual is comprised of a variety of bones of differing lengths. The largest contribution to the lengthening of a skeletal element, and to the differential elongation of elements, comes from a dramatic increase in the volume of hypertrophic chondrocytes in the growth plate as they undergo terminal differentiation. However, the mechanisms of chondrocyte volume enlargement have remained a mystery. Here we use quantitative phase microscopy to show that mammalian chondrocytes undergo three distinct phases of volume increase, including a phase of massive cell swelling in which the cellular dry mass is significantly diluted. In light of the tight fluid regulatory mechanisms known to control volume in many cell types, this is a remarkable mechanism for increasing cell size and regulating growth rate. It is, however, the duration of the final phase of volume enlargement by proportional dry mass increase at low density that varies most between rapidly and slowly elongating growth plates. Moreover, we find that this third phase is locally regulated through a mechanism dependent on insulin-like growth factor. This study provides a framework for understanding how skeletal size is regulated and for exploring how cells sense, modify and establish a volume set point.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

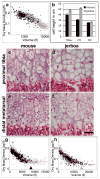
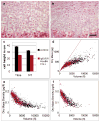
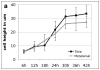
References
-
- Wilsman NJ, Farnum CE, Leiferman EM, Fry M, Barreto C. Differential growth by growth plates as a function of multiple parameters of chondrocytic kinetics. J Orthop Res. 1996;14:927–36. - PubMed
-
- Hunziker EB, Schenk RK, Cruz-Orive LM. Quantitation of chondrocyte performance in growth-plate cartilage during longitudinal bone growth. J Bone Joint Surg Am. 1987;69:162–173. - PubMed
-
- Breur GJ, VanEnkevort BA, Farnum CE, Wilsman NJ. Linear relationship between the volume of hypertrophic chondrocytes and the rate of longitudinal bone growth in growth plates. J Orthop Res. 1991;9:348–359. - PubMed
-
- Kuhn JL, Delacey JH, Leenellett EE. Relationship between bone growth rate and hypertrophic chondrocyte volume in new zealand white rabbits of varying ages. Journal of Orthopaedic Research. 1996;14:706–711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

