Synthesis and antimicrobial activity of novel substituted ethyl 2-(quinolin-4-yl)-propanoates
- PMID: 23486102
- PMCID: PMC6270033
- DOI: 10.3390/molecules18033227
Synthesis and antimicrobial activity of novel substituted ethyl 2-(quinolin-4-yl)-propanoates
Abstract
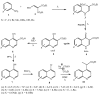
Substituted 4-hydroxyquinolines were synthesized from anilines and diethyl 2-(ethoxymethylene)malonate by the Gould-Jacobs reaction via cyclization of the intermediate anilinomethylenemalonate followed by hydrolysis and decarboxylation. The 4-hydroxyquinolines reacted with phosphorous oxychloride to form 4-chloroquinolines, which reacted on heating with diethyl sodiomethylmalonate in DMF to yield moderate yields of substituted ethyl 2-(quinolin-4-yl)propanoates, many of which showed potent antimicrobial activity against Helicobacter pylori.
Figures




References
-
- Williamson W.R.N., editor. Anti-Inflammatory Compounds. Marcel Dekker; New York, NY, USA: 1987. p. 250.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

