Design, synthesis and biological evaluation of N-sulfonyl homoserine lactone derivatives as inhibitors of quorum sensing in Chromobacterium violaceum
- PMID: 23486105
- PMCID: PMC6270181
- DOI: 10.3390/molecules18033266
Design, synthesis and biological evaluation of N-sulfonyl homoserine lactone derivatives as inhibitors of quorum sensing in Chromobacterium violaceum
Abstract
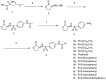
A novel series of N-sulfonyl homoserine lactone derivatives 5a-l has been designed, synthesized and evaluated for quorum sensing inhibitory activities towards violacein production. Of the compounds synthesized, compound 5h was found to possess an excellent level of enantiopurity (99.2% e.e.). The results indicated that compounds bearing an ortho substituent on their phenyl ring exhibited excellent levels of inhibitory activity against violacein production. Compounds 5h and 5k in particular, with IC₅₀ values of 1.64 and 1.66 µM, respectively, were identified as promising lead compounds for further structural modification.
Figures
Similar articles
-
Targeting quorum sensing by designing azoline derivatives to inhibit the N-hexanoyl homoserine lactone-receptor CviR: Synthesis as well as biological and theoretical evaluations.Bioorg Med Chem. 2015 Dec 15;23(24):7565-77. doi: 10.1016/j.bmc.2015.10.046. Epub 2015 Nov 2. Bioorg Med Chem. 2015. PMID: 26654469
-
Synthesis and Biological Evaluation of Novel L-Homoserine Lactone Analogs as Quorum Sensing Inhibitors of Pseudomonas aeruginosa.Chem Pharm Bull (Tokyo). 2019;67(10):1088-1098. doi: 10.1248/cpb.c19-00359. Chem Pharm Bull (Tokyo). 2019. PMID: 31582628
-
[The effects of alkylhydroxybenzenes on homoserine lactone-induced manifestations of quorum sensing in bacteria].Prikl Biokhim Mikrobiol. 2014 Jul-Aug;50(4):391-7. doi: 10.7868/s0555109914040199. Prikl Biokhim Mikrobiol. 2014. PMID: 25707115 Russian.
-
Next generation quorum sensing inhibitors: Accounts on structure activity relationship studies and biological activities.Bioorg Med Chem. 2020 Nov 1;28(21):115728. doi: 10.1016/j.bmc.2020.115728. Epub 2020 Aug 29. Bioorg Med Chem. 2020. PMID: 33065436 Review.
-
Microbial synthesis of violacein pigment and its potential applications.Crit Rev Biotechnol. 2021 Sep;41(6):879-901. doi: 10.1080/07388551.2021.1892579. Epub 2021 Mar 17. Crit Rev Biotechnol. 2021. PMID: 33730942 Review.
Cited by
-
Quorum Sensing Inhibitors: Curbing Pathogenic Infections through Inhibition of Bacterial Communication.Iran J Pharm Res. 2021 Spring;20(2):486-514. doi: 10.22037/ijpr.2020.113470.14318. Iran J Pharm Res. 2021. PMID: 34567177 Free PMC article. Review.
-
Quorum Sensing Signal Selectivity and the Potential for Interspecies Cross Talk.mBio. 2019 Mar 5;10(2):e00146-19. doi: 10.1128/mBio.00146-19. mBio. 2019. PMID: 30837333 Free PMC article.
-
Short Synthesis of Structurally Diverse N-Acylhomoserine Lactone Analogs and Discovery of Novel Quorum Quenchers Against Gram-Negative Pathogens.Int J Mol Sci. 2025 Feb 19;26(4):1775. doi: 10.3390/ijms26041775. Int J Mol Sci. 2025. PMID: 40004238 Free PMC article.
-
Design, Synthesis, and Evaluation of Alkyl-Quinoxalin-2(1H)-One Derivatives as Anti-Quorum Sensing Molecules, Inhibiting Biofilm Formation in Aeromonas caviae Sch3.Molecules. 2018 Nov 24;23(12):3075. doi: 10.3390/molecules23123075. Molecules. 2018. PMID: 30477243 Free PMC article.
-
A Bacterial Isolate Capable of Quenching Both Diffusible Signal Factor- and N-Acylhomoserine Lactone-Family Quorum Sensing Signals Shows Much Enhanced Biocontrol Potencies.J Agric Food Chem. 2022 Jun 29;70(25):7716-7726. doi: 10.1021/acs.jafc.2c01299. Epub 2022 Jun 16. J Agric Food Chem. 2022. PMID: 35708354 Free PMC article.
References
-
- Geske G.D., O’Nell J.C., Miller D.M., Mattmann M.E., Blackwell H.E. Modulation of Bacterial Quorum Sensing with Synthetic Ligands: Systematic Evaluation of N-Acylated Homoserine Lactones in Multiple Species and New Insights into Their Mechanisms of Action. J. Am. Chem. Soc. 2007;129:13613–13625. - PMC - PubMed
-
- Persson T., Hansen T.H., Rasmussen T.B., Skindersø M.E., Givskov M., Nielsen J. Rational design and synthesis of new quorum-sensing inhibitors derived from acylated homoserine lactones and natural products from garlic. Org. Biomol. Chem. 2005;3:253–262. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources