Design, synthesis and biological evaluation of N-sulfonyl homoserine lactone derivatives as inhibitors of quorum sensing in Chromobacterium violaceum
- PMID: 23486105
- PMCID: PMC6270181
- DOI: 10.3390/molecules18033266
Design, synthesis and biological evaluation of N-sulfonyl homoserine lactone derivatives as inhibitors of quorum sensing in Chromobacterium violaceum
Abstract
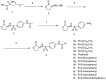
A novel series of N-sulfonyl homoserine lactone derivatives 5a-l has been designed, synthesized and evaluated for quorum sensing inhibitory activities towards violacein production. Of the compounds synthesized, compound 5h was found to possess an excellent level of enantiopurity (99.2% e.e.). The results indicated that compounds bearing an ortho substituent on their phenyl ring exhibited excellent levels of inhibitory activity against violacein production. Compounds 5h and 5k in particular, with IC₅₀ values of 1.64 and 1.66 µM, respectively, were identified as promising lead compounds for further structural modification.
Figures
References
-
- Geske G.D., O’Nell J.C., Miller D.M., Mattmann M.E., Blackwell H.E. Modulation of Bacterial Quorum Sensing with Synthetic Ligands: Systematic Evaluation of N-Acylated Homoserine Lactones in Multiple Species and New Insights into Their Mechanisms of Action. J. Am. Chem. Soc. 2007;129:13613–13625. - PMC - PubMed
-
- Persson T., Hansen T.H., Rasmussen T.B., Skindersø M.E., Givskov M., Nielsen J. Rational design and synthesis of new quorum-sensing inhibitors derived from acylated homoserine lactones and natural products from garlic. Org. Biomol. Chem. 2005;3:253–262. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources