Mesenchymal stem cells ameliorate experimental peritoneal fibrosis by suppressing inflammation and inhibiting TGF-β1 signaling
- PMID: 23486522
- PMCID: PMC3731556
- DOI: 10.1038/ki.2013.81
Mesenchymal stem cells ameliorate experimental peritoneal fibrosis by suppressing inflammation and inhibiting TGF-β1 signaling
Abstract
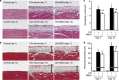
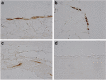
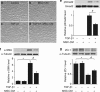
Mesenchymal stem cells (MSCs) are multipotent adult stem cells that have regenerative capability and exert paracrine actions on damaged tissues. Since peritoneal fibrosis is a serious complication of peritoneal dialysis, we tested whether MSCs suppress this using a chlorhexidine gluconate model in rats. Although MSCs isolated from green fluorescent protein-positive rats were detected for only 3 days following their injection, immunohistochemical staining showed that MSCs suppressed the expression of mesenchymal cells, their effects on the deposition of extracellular matrix proteins, and the infiltration of macrophages for 14 days. Moreover, MSCs reduced the functional impairment of the peritoneal membrane. Cocultures of MSCs and human peritoneal mesothelial cells using a Transwell system indicated that the beneficial effects of MSCs on the glucose-induced upregulation of transforming growth factor-β1(TGF-β1) and fibronectin mRNA expression in the human cells were likely due to paracrine actions. Preincubation in MSC-conditioned medium suppressed TGF-β1-induced epithelial-to-mesenchymal transition, α-smooth muscle actin, and the decrease in zonula occludens-1 in cultured human peritoneal mesothelial cells. Although bone morphogenic protein 7 was not detected, MSCs secreted hepatocyte growth factor and a neutralizing antibody to this inhibited TGF-β1 signaling. Thus, our findings imply that MSCs ameliorate experimental peritoneal fibrosis by suppressing inflammation and TGF-β1 signaling in a paracrine manner.
Figures







Similar articles
-
Mesenchymal stem cells attenuate peritoneal injury through secretion of TSG-6.PLoS One. 2012;7(8):e43768. doi: 10.1371/journal.pone.0043768. Epub 2012 Aug 17. PLoS One. 2012. PMID: 22912904 Free PMC article.
-
Mesenchymal stem cells cultured in serum-free medium ameliorate experimental peritoneal fibrosis.Stem Cell Res Ther. 2021 Mar 23;12(1):203. doi: 10.1186/s13287-021-02273-1. Stem Cell Res Ther. 2021. PMID: 33757592 Free PMC article.
-
Nitro-oleic acid inhibits the high glucose-induced epithelial-mesenchymal transition in peritoneal mesothelial cells and attenuates peritoneal fibrosis.Am J Physiol Renal Physiol. 2020 Feb 1;318(2):F457-F467. doi: 10.1152/ajprenal.00425.2019. Epub 2019 Nov 25. Am J Physiol Renal Physiol. 2020. PMID: 31760768
-
Reprogramming of Mesothelial-Mesenchymal Transition in Chronic Peritoneal Diseases by Estrogen Receptor Modulation and TGF-β1 Inhibition.Int J Mol Sci. 2020 Jun 10;21(11):4158. doi: 10.3390/ijms21114158. Int J Mol Sci. 2020. PMID: 32532126 Free PMC article. Review.
-
Clinical and preclinical studies of mesenchymal stem cells to alleviate peritoneal fibrosis.Stem Cell Res Ther. 2024 Jul 30;15(1):237. doi: 10.1186/s13287-024-03849-3. Stem Cell Res Ther. 2024. PMID: 39080683 Free PMC article. Review.
Cited by
-
TGF-β1 promotes expression of fibrosis-related genes through the induction of histone variant H3.3 and histone chaperone HIRA.Sci Rep. 2018 Sep 19;8(1):14060. doi: 10.1038/s41598-018-32518-8. Sci Rep. 2018. PMID: 30232404 Free PMC article.
-
MSC-secreted TGF-β regulates lipopolysaccharide-stimulated macrophage M2-like polarization via the Akt/FoxO1 pathway.Stem Cell Res Ther. 2019 Nov 26;10(1):345. doi: 10.1186/s13287-019-1447-y. Stem Cell Res Ther. 2019. PMID: 31771622 Free PMC article.
-
Extracellular Vesicles Derived from Adipose Mesenchymal Stem Cells Promote Peritoneal Healing by Activating MAPK-ERK1/2 and PI3K-Akt to Alleviate Postoperative Abdominal Adhesion.Stem Cells Int. 2022 May 5;2022:1940761. doi: 10.1155/2022/1940761. eCollection 2022. Stem Cells Int. 2022. PMID: 35578661 Free PMC article.
-
How to Improve the Biocompatibility of Peritoneal Dialysis Solutions (without Jeopardizing the Patient's Health).Int J Mol Sci. 2021 Jul 26;22(15):7955. doi: 10.3390/ijms22157955. Int J Mol Sci. 2021. PMID: 34360717 Free PMC article. Review.
-
Enhanced renoprotective effect of GDNF-modified adipose-derived mesenchymal stem cells on renal interstitial fibrosis.Stem Cell Res Ther. 2021 Jan 7;12(1):27. doi: 10.1186/s13287-020-02049-z. Stem Cell Res Ther. 2021. PMID: 33413640 Free PMC article.
References
-
- Dobbie J. Morphology of the peritoneum in CAPD. Blood Purif. 1989;7:74–85. - PubMed
-
- Nakamoto H, Kawaguchi Y, Suzuki H. Encapsulating peritoneal sclerosis in patients undergoing continuous ambulatory peritoneal dialysis in Japan. Adv Perit Dial. 2002;18:119–123. - PubMed
-
- Margetts P, Bonniaud P. Basic mechanisms and clinical implications of peritoneal fibrosis. Perit Dial Int. 2003;23:530–541. - PubMed
-
- Mortier S, De Vriese A, Van de Voorde J, et al. Hemodynamic effects of peritoneal dialysis solutions on the rat peritoneal membrane: role of acidity, buffer choice, glucose concentration, and glucose degradation products. J Am Soc Nephrol. 2002;13:480–489. - PubMed
-
- Williams J, Craig K, Topley N, et al. Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol. 2002;13:470–479. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

