Molecular and pathologic insights from latent HIV-1 infection in the human brain
- PMID: 23486877
- PMCID: PMC3662272
- DOI: 10.1212/WNL.0b013e31828c2e9e
Molecular and pathologic insights from latent HIV-1 infection in the human brain
Abstract
Objective: We aimed to investigate whether HIV latency in the CNS might have adverse molecular, pathologic, and clinical consequences.
Methods: This was a case-control comparison of HIV-1 seropositive (HIV+) patients with clinical and neuropathologic examination. Based on the levels of HIV-1 DNA, RNA, and p24 in the brain, cases were classified as controls, latent HIV CNS infection, and HIV encephalitis (HIVE). Analysis of epigenetic markers including BCL11B, neurodegeneration, and neuroinflammation was performed utilizing immunoblot, confocal microscopy, immunochemistry/image analysis, and qPCR. Detailed antemortem neurocognitive data were available for 23 out of the 32 cases.
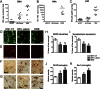
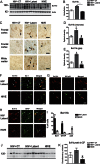
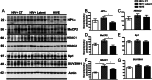
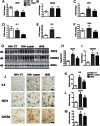
Results: HIV+ controls (n = 12) had no detectable HIV-1 DNA, RNA, or p24 in the CNS; latent HIV+ cases (n = 10) showed high levels of HIV-1 DNA but no HIV RNA or p24; and HIVE cases (n = 10) had high levels of HIV-1 DNA, RNA, and p24. Compared to HIV+ controls, the HIV+ latent cases displayed moderate cognitive impairment with neurodegenerative and neuroinflammatory alterations, although to a lesser extent than HIVE cases. Remarkably, HIV+ latent cases showed higher levels of BCL11B and other chromatin modifiers involved in silencing. Increased BCL11B was associated with deregulation of proinflammatory genes like interleukin-6, tumor necrosis factor-α, and CD74.
Conclusion: Persistence of latent HIV-1 infection in the CNS was associated with increased levels of chromatin modifiers, including BCL11B. Alteration of these epigenetic factors might result in abnormal transcriptomes, leading to inflammation, neurodegeneration, and neurocognitive impairment. BCL11B and other epigenetic factors involved in silencing might represent potential targets for HIV-1 involvement of the CNS.
Figures
Comment in
-
The cost of silencing HIV in the brain.Neurology. 2013 Apr 9;80(15):1363-4. doi: 10.1212/WNL.0b013e31828c3077. Epub 2013 Mar 13. Neurology. 2013. PMID: 23486870 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- AG03197/AG/NIA NIH HHS/United States
- R01 AG043384/AG/NIA NIH HHS/United States
- U24 MH100928/MH/NIMH NIH HHS/United States
- U01 MH83506/MH/NIMH NIH HHS/United States
- AG5131/AG/NIA NIH HHS/United States
- NS057096/NS/NINDS NIH HHS/United States
- MH79881/MH/NIMH NIH HHS/United States
- MH5974/MH/NIMH NIH HHS/United States
- P30 NS076411/NS/NINDS NIH HHS/United States
- DA12065/DA/NIDA NIH HHS/United States
- R01 MH062962/MH/NIMH NIH HHS/United States
- MH076681/MH/NIMH NIH HHS/United States
- P30 MH062512/MH/NIMH NIH HHS/United States
- U01 MH083506/MH/NIMH NIH HHS/United States
- MH62512/MH/NIMH NIH HHS/United States
- MH62962/MH/NIMH NIH HHS/United States
- MH58164/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
