Spontaneous and task-evoked brain activity negatively interact
- PMID: 23486941
- PMCID: PMC3637953
- DOI: 10.1523/JNEUROSCI.2922-12.2013
Spontaneous and task-evoked brain activity negatively interact
Abstract
A widely held assumption is that spontaneous and task-evoked brain activity sum linearly, such that the recorded brain response in each single trial is the algebraic sum of the constantly changing ongoing activity and the stereotypical evoked activity. Using functional magnetic resonance imaging signals acquired from normal humans, we show that this assumption is invalid. Across widespread cortices, evoked activity interacts negatively with ongoing activity, such that higher prestimulus baseline results in less activation or more deactivation. As a consequence of this negative interaction, trial-to-trial variability of cortical activity decreases following stimulus onset. We further show that variability reduction follows overlapping but distinct spatial pattern from that of task-activation/deactivation and it contains behaviorally relevant information. These results favor an alternative perspective to the traditional dichotomous framework of ongoing and evoked activity. That is, to view the brain as a nonlinear dynamical system whose trajectory is tighter when performing a task. Further, incoming sensory stimuli modulate the brain's activity in a manner that depends on its initial state. We propose that across-trial variability may provide a new approach to brain mapping in the context of cognitive experiments.
Figures



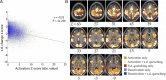

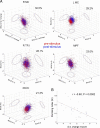


Comment in
-
Interactions between resting-state and task-evoked brain activity suggest a different approach to fMRI analysis.J Neurosci. 2013 Aug 7;33(32):12912-4. doi: 10.1523/JNEUROSCI.2580-13.2013. J Neurosci. 2013. PMID: 23926247 Free PMC article. No abstract available.
References
-
- Abbott LF, Rajan K, Sompolinksy H. Interactions between intrinsic and stimulus-dependent activity in recurrent neural networks. In: Ding M, Glanzman D, editors. The dynamic brain: an exploration of neuronal variability and its functional significance. New York: Oxford UP; 2011.
-
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. - PubMed
-
- Bandettini PA, Ungerleider LG. From neuron to BOLD: new connections. Nat Neurosci. 2001;4:864–866. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
