The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for Basal Ganglia models and deep brain stimulation
- PMID: 23486951
- PMCID: PMC3755746
- DOI: 10.1523/JNEUROSCI.4674-12.2013
The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for Basal Ganglia models and deep brain stimulation
Abstract
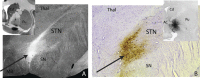
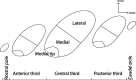
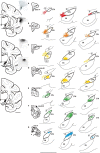
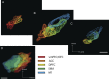
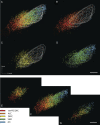
The identification of a hyperdirect cortico-subthalamic nucleus connection highlighted the important role of the subthalamic nucleus (STN) in regulating behavior. However, this pathway was shown primarily from motor areas. Hyperdirect pathways associated with cognitive and motivational cortical regions are particularly relevant given recent data from deep brain stimulation, both for neurologic and psychiatric disorders. Our experiments were designed to demonstrate the existence and organization of prefrontal-STN projections, help delineate the "limbic" STN, and determine whether convergence between cortico-STN fibers from functionally diverse cortical areas exists in the STN. We injected anterograde tracers in the ventromedial prefrontal, orbitofrontal, anterior cingulate, and dorsal prefrontal cortices of Macaca nemestrina and Macaca fascicularis to analyze the organization of terminals and passing fibers in the STN. Results show a topographically organized prefrontal hyperdirect pathway in primates. Limbic areas project to the medial tip of the nucleus, straddling its border and extending into the lateral hypothalamus. Associative areas project to the medial half, motor areas to the lateral half. Limbic projections terminated primarily rostrally and motor projections more caudally. The extension of limbic projections into the lateral hypothalamus, suggests that this region be included in the STN. A high degree of convergence exists between projections from functionally diverse cortical areas, creating potentially important interfaces between terminal fields. Taken together, the results provide an anatomical substrate to extend the role of the hyperdirect pathway in models of basal ganglia function, and new keys for understanding deep brain stimulation effects on cognitive and motivational aspects of behavior.
Figures


Comment in
-
Subdivisions and anatomical boundaries of the subthalamic nucleus.J Neurosci. 2013 May 29;33(22):9233-4. doi: 10.1523/JNEUROSCI.1266-13.2013. J Neurosci. 2013. PMID: 23719792 Free PMC article. No abstract available.
-
Prefrontal--STN projections, the highway for emotion and cognition control.Mov Disord. 2014 Mar;29(3):305. doi: 10.1002/mds.25760. Epub 2013 Dec 20. Mov Disord. 2014. PMID: 24375493 No abstract available.
References
-
- Bogacz R, Larsen T. Integration of reinforcement learning and optimal decision-making theories of the basal ganglia. Neural computation. 2011;23:817–851. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
